Calcium Metabolism and Breast Cancer: Echoes of Lactation?
- PMID: 33299957
- PMCID: PMC7720883
- DOI: 10.1016/j.coemr.2020.11.006
Calcium Metabolism and Breast Cancer: Echoes of Lactation?
Abstract
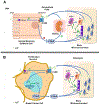
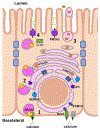
Lactation requires a series of adaptations in maternal calcium and bone metabolism to ensure a steady supply of calcium to the lactating mammary gland. The alterations in systemic metabolism are accompanied by alterations in the expression of calcium receptors, channels, binding proteins, pumps and transporters in mammary epithelial cells to increase the uptake of calcium from the extracellular fluid and to transport it into milk. Intracellular calcium regulates signaling pathways that mediate changes in cell proliferation, differentiation and death and many of the molecules involved in supporting and coordinating calcium secretion into milk are re-expressed and redeployed to support malignant behavior in breast cancer cells. In this article, we review adaptations of systemic calcium homeostasis during lactation, as well as the mechanisms of milk calcium transport. We then discuss how reactivation of these pathways contributes to the pathophysiology of breast cancer.
Keywords: Bone; Breast Cancer; Calcium-sensing receptor; Lactation; Mammary gland; Parathyroid hormone-related protein.
Figures

References
-
- Sadovnikova A, Wysolmerski John J, Hovey Russell C. The onset and maintenance of human lactation and its endocrine regulation. In: Kovacs C, Deal C, eds. 1st Edition ed: Academic Press; 2019:189–200.
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. - PubMed
-
- Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiological Reviews. 2016;96(2):449–547. - PubMed
-
This is an extensive review of the maternal adaptations in mineral and bone physiology during pregnancy, lactation and post-weaning recovery
-
- Ross AC, Manson JAE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. Vol 96: Endocrine Society; 2011:53–58. - PMC - PubMed
-
- Torres DA, Freitas MB, Gonçalves RV. Changes in bone turnover and calcium homeostasis during pregnancy and lactation in mammals: a meta-analysis. Reproduction, Fertility and Development. 2018;30(5):681–681. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
