Neutrophil Elastase Facilitates Tumor Cell Intravasation and Early Metastatic Events
- PMID: 33299970
- PMCID: PMC7702017
- DOI: 10.1016/j.isci.2020.101799
Neutrophil Elastase Facilitates Tumor Cell Intravasation and Early Metastatic Events
Abstract
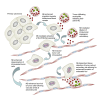
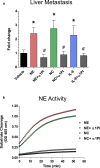
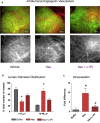
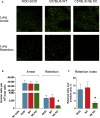
Functional roles of neutrophil elastase (NE) have not been examined in distinct steps of the metastatic cascade. NE, delivered to primary tumors as a purified enzyme or within intact neutrophils or neutrophil granule content, enhanced human tumor cell intravasation and subsequent dissemination via NE-mediated formation of dilated intratumoral vasculature. These effects depended on picomole range of NE activity, sensitive to its natural inhibitor, α1PI. In Elane-negative mice, the lack of NE decreased lung retention of human tumor cells in experimental metastasis. Furthermore, NE was essential for spontaneous metastasis of murine carcinoma cells in a syngeneic orthotopic model of oral cancer. NE also induced tumor cell survival and migration via Src/PI3K-dependent activation of Akt signaling, vital for tumor cell dissemination in vivo. Together, our findings implicate NE, a potent host enzyme specific for first-responding innate immune cells, as directly involved in early metastatic events and a potential target for therapeutic intervention.
Keywords: Cancer; Cell Biology.
© 2020 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Aikawaa N., Ishizaka A., Hirasawa H., Shimazaki S., Yamamoto Y., Sugimoto H., Shinozaki M., Taenaka N., Endo S., Ikeda T. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm. Pharmacol. Ther. 2011;24:549–554. - PubMed
-
- Altomare D.A., Testa J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

