This is a preprint.
Treatment of Severe COVID-19 with Convalescent Plasma in the Bronx, NYC
- PMID: 33300012
- PMCID: PMC7724683
- DOI: 10.1101/2020.12.02.20242909
Treatment of Severe COVID-19 with Convalescent Plasma in the Bronx, NYC
Update in
-
Treatment of severe COVID-19 with convalescent plasma in Bronx, NYC.JCI Insight. 2021 Feb 22;6(4):e142270. doi: 10.1172/jci.insight.142270. JCI Insight. 2021. PMID: 33476300 Free PMC article.
Abstract
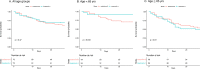
Convalescent plasma with severe acute respiratory disease coronavirus 2 (SARS-CoV-2) antibodies (CCP) may hold promise as treatment for Coronavirus Disease 2019 (COVID-19). We compared the mortality and clinical outcome of patients with COVID-19 who received 200mL of CCP with a Spike protein IgG titer ≥1:2,430 (median 1:47,385) within 72 hours of admission to propensity score-matched controls cared for at a medical center in the Bronx, between April 13 to May 4, 2020. Matching criteria for controls were age, sex, body mass index, race, ethnicity, comorbidities, week of admission, oxygen requirement, D-dimer, lymphocyte counts, corticosteroids, and anticoagulation use. There was no difference in mortality or oxygenation between CCP recipients and controls at day 28. When stratified by age, compared to matched controls, CCP recipients <65 years had 4-fold lower mortality and 4-fold lower deterioration in oxygenation or mortality at day 28. For CCP recipients, pre-transfusion Spike protein IgG, IgM and IgA titers were associated with mortality at day 28 in univariate analyses. No adverse effects of CCP were observed. Our results suggest CCP may be beneficial for hospitalized patients <65 years, but data from controlled trials is needed to validate this finding and establish the effect of ageing on CCP efficacy.
Conflict of interest statement
Conflicts of Interest statement KC is a member of the Scientific Advisory Board of Integrum Scientific, LLC. In addition, KC has a SARS-CoV-2 spike neutralization assay patent pending, and a SARS-CoV-2 spike antibody assay patent pending. JL reports grants from Adimab LLC, grants from Integrated BioTherapeutics, grants from Mapp Biopharmaceutical, personal fees from Johnson & Johnson, personal fees from Celdara Medical. In addition, JL has a COVID-19 antibody diagnostic patent pending. Other authors have declared that no conflict of interest exists.
Figures



References
-
- John Hopkins University Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. Accessed Nov 16. https://coronavirus.jhu.edu/map.html.
Publication types
Grants and funding
- T32 GM007491/GM/NIGMS NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- UL1 TR002377/TR/NCATS NIH HHS/United States
- R21 AI141367/AI/NIAID NIH HHS/United States
- R01 AI143453/AI/NIAID NIH HHS/United States
- R01 AI125462/AI/NIAID NIH HHS/United States
- UL1 TR001073/TR/NCATS NIH HHS/United States
- P30 AI124414/AI/NIAID NIH HHS/United States
- UL1 TR002556/TR/NCATS NIH HHS/United States
- U19 AI142777/AI/NIAID NIH HHS/United States
- R01 AI145024/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
