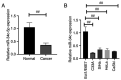miR‑34c‑5p targets Notch1 and suppresses the metastasis and invasion of cervical cancer
- PMID: 33300051
- PMCID: PMC7751466
- DOI: 10.3892/mmr.2020.11759
miR‑34c‑5p targets Notch1 and suppresses the metastasis and invasion of cervical cancer
Abstract
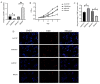
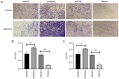
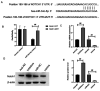
Micro (mi)RNAs are crucial participants in the progression of cervical cancer (CC). Growing evidence indicates that miRNA (miR)‑34c‑5p is a pivotal tumor suppressor in numerous types of cancer and its functions in CC require further investigating. The present study demonstrated that there was a decreased level of miR‑34c‑5p in CC‑associated cell lines compared with healthy control samples. It also demonstrated that miR‑34c‑5p targeted Notch1 and suppressed CC progression. Dual‑luciferase reporter assays verified the targeted relationship of miR‑34c‑5p and Notch1. The expression of Notch1 in HeLa cells was markedly reduced following miR‑34c‑5p overexpression and the proliferation, migration and invasion of HeLa cells were reduced although apoptosis was accelerated. However, overexpression of miR‑34c‑5p was reversed following the addition of Notch1, which supported the finding of the targeted relationship between miR‑34c‑5p and Notch1. Flow cytometry demonstrated that miR‑34c‑5p inhibited the proliferation of HeLa cells while accelerating apoptosis. The present study concluded that miR‑34c‑5p was a tumor suppressor in CC and may be a novel measure for the future treatment of CC.
Figures