Basophil activation test shows high accuracy in the diagnosis of peanut and tree nut allergy: The Markers of Nut Allergy Study
- PMID: 33300157
- PMCID: PMC8608143
- DOI: 10.1111/all.14695
Basophil activation test shows high accuracy in the diagnosis of peanut and tree nut allergy: The Markers of Nut Allergy Study
Abstract
Background: Peanut and tree nut allergies are the most important causes of anaphylaxis. Co-reactivity to more than one nut is frequent, and co-sensitization in the absence of clinical data is often obtained. Confirmatory oral food challenges (OFCs) are inconsistently performed.
Objective: To investigate the utility of the basophil activation test (BAT) in diagnosing peanut and tree nut allergies.
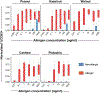
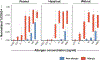
Methods: The Markers Of Nut Allergy Study (MONAS) prospectively enrolled patients aged 0.5-17 years with confirmed peanut and/or tree nut (almond, cashew, hazelnut, pistachio, walnut) allergy or sensitization from Canadian (n = 150) and Austrian (n = 50) tertiary pediatric centers. BAT using %CD63+ basophils (SSClow/CCR3pos) as outcome was performed with whole blood samples stimulated with allergen extracts of each nut (0.001-1000 ng/mL protein). BAT results were assessed against confirmed allergic status in a blinded fashion to develop a generalizable statistical model for comparison to extract and marker allergen-specific IgE.
Results: A mixed effect model integrating BAT results for 10 and 100 ng/mL of peanut and individual tree nut extracts was optimal. The area under the ROC curve (AUROC) was 0.98 for peanut, 0.97 for cashew, 0.92 for hazelnut, 0.95 for pistachio, and 0.97 for walnut. The BAT outperformed sIgE testing for peanut or hazelnut and was comparable for walnut (AUROC 0.95, 0.94, 0.92) in a sub-analysis in sensitized patients undergoing OFC.
Conclusions: Basophil activation test can predict allergic clinical status to peanut and tree nuts in multi-nut-sensitized children and may reduce the need for high-risk OFCs in patients.
Keywords: allergy diagnosis; basophil; challenge tests; food allergy; molecular allergology; multiple nut allergy; pediatrics.
© 2020 EAACI and John Wiley and Sons A/S. Published by John Wiley and Sons Ltd.
Figures
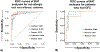
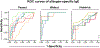
References
-
- Ben-Shoshan M, Harrington DW, Soller L, et al. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. Journal of Allergy and Clinical Immunology. 2010;125(6):1327–1335. - PubMed
-
- McWilliam V, Koplin J, Lodge C, Tang M, Dharmage S, Allen K. The Prevalence of Tree Nut Allergy: A Systematic Review. Current Allergy and Asthma Reports. 2015;15(9):54. - PubMed
-
- Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. Journal of Allergy and Clinical Immunology. 2010;125(6):1322–1326. - PubMed
-
- Venter C, Hasan Arshad S, Grundy J, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK: Time trends in peanut allergy. Allergy. 2010;65(1):103–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

