Whom Should We Test for COVID-19? Performance of a Symptom and Risk Factor Questionnaire on COVID-19 Test Results and Patient Outcomes in an Immediate Care Setting
- PMID: 33300408
- PMCID: PMC7734557
- DOI: 10.1177/2150132720981297
Whom Should We Test for COVID-19? Performance of a Symptom and Risk Factor Questionnaire on COVID-19 Test Results and Patient Outcomes in an Immediate Care Setting
Abstract
Introduction: The CDC and Illinois Department of Public Health disseminated risk factor criteria for COVID-19 testing early in the pandemic. The objective of this study is to assess the effectiveness of risk stratifying patients for COVID-19 testing and to identify which risk factors and which other clinical variables were associated with SARS-CoV-2 PCR test positivity.
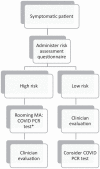
Methods: We conducted an observational cohort study on a sample of symptomatic patients evaluated at an immediate care setting. A risk assessment questionnaire was administered to every patient before clinician evaluation. High-risk patients received SARS-CoV-2 test and low-risk patients were evaluated by a clinician and selectively tested based on clinician judgment. Multivariate analyses tested whether risk factors and additional variables were associated with test positivity.
Results: The adjusted odds ratio of testing positive was associated with COVID-19-positive or suspect close contact (aOR 1.56, 95% CI 1.15-2.10), large gathering attendance with a COVID-19-positive individual (aOR 1.92, 95% CI 1.10-3.34), and, with the largest effect size, decreased taste/smell (aOR 2.83, 95% CI 2.01-3.99). Testing positive was associated with ages 45-64 and ≥65 (aOR 1.75, 95% CI 1.25-2.44, and aOR 2.78, 95% CI 1.49-5.16), systolic blood pressures ≤120 (aOR 1.64, 95% CI 1.20-2.24), and, with the largest effect size, temperatures ≥99.0°F (aOR 3.06, 95% CI 2.23-4.20). The rate of positive SARS-CoV-2 test was similar between high-risk and low risk patients (225 [22.2%] vs 50 [19.8%]; P = .41).
Discussion: The risk assessment questionnaire was not effective at stratifying patients for testing. Although individual risk factors were associated with SARS-CoV-2 test positivity, the low-risk group had similar positivity rates to the high-risk group. Our observations underscore the need for clinicians to develop clinical experience and share best practices and for systems and payors to support policies, funding, and resources to test all symptomatic patients.
Keywords: COVID-19; diagnostic testing; infectious disease; primary care; quality improvement.
Conflict of interest statement
Figures
References
-
- Illinois Department of Public Health. IDPH Interim COVID-19 Guidance. Accessed March 19, 2020 https://www.dph.illinois.gov/sites/default/files/COVID19/20200319_COVID1....
-
- Appleby J. Why it takes so long to get most COVID-19 test results. Accessed October 5, 2020 https://www.npr.org/sections/health-shots/2020/03/28/822869504/why-it-ta....
-
- NorthShore becomes first local hospital network to conduct its own COVID-19 testing [Transcript]. WGN TV. March 17, 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous