Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial
- PMID: 33300950
- PMCID: PMC7729589
- DOI: 10.1001/jama.2020.23370
Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial
Erratum in
-
Incorrect y-Axis and Panel Labels in Figure.JAMA. 2022 May 17;327(19):1929. doi: 10.1001/jama.2022.7048. JAMA. 2022. PMID: 35452086 Free PMC article. No abstract available.
Abstract
Importance: Among all subtypes of breast cancer, triple-negative breast cancer has a relatively high relapse rate and poor outcome after standard treatment. Effective strategies to reduce the risk of relapse and death are needed.
Objective: To evaluate the efficacy and adverse effects of low-dose capecitabine maintenance after standard adjuvant chemotherapy in early-stage triple-negative breast cancer.
Design, setting, and participants: Randomized clinical trial conducted at 13 academic centers and clinical sites in China from April 2010 to December 2016 and final date of follow-up was April 30, 2020. Patients (n = 443) had early-stage triple-negative breast cancer and had completed standard adjuvant chemotherapy.
Interventions: Eligible patients were randomized 1:1 to receive capecitabine (n = 222) at a dose of 650 mg/m2 twice a day by mouth for 1 year without interruption or to observation (n = 221) after completion of standard adjuvant chemotherapy.
Main outcomes and measures: The primary end point was disease-free survival. Secondary end points included distant disease-free survival, overall survival, locoregional recurrence-free survival, and adverse events.
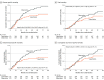
Results: Among 443 women who were randomized, 434 were included in the full analysis set (mean [SD] age, 46 [9.9] years; T1/T2 stage, 93.1%; node-negative, 61.8%) (98.0% completed the trial). After a median follow-up of 61 months (interquartile range, 44-82), 94 events were observed, including 38 events (37 recurrences and 32 deaths) in the capecitabine group and 56 events (56 recurrences and 40 deaths) in the observation group. The estimated 5-year disease-free survival was 82.8% in the capecitabine group and 73.0% in the observation group (hazard ratio [HR] for risk of recurrence or death, 0.64 [95% CI, 0.42-0.95]; P = .03). In the capecitabine group vs the observation group, the estimated 5-year distant disease-free survival was 85.8% vs 75.8% (HR for risk of distant metastasis or death, 0.60 [95% CI, 0.38-0.92]; P = .02), the estimated 5-year overall survival was 85.5% vs 81.3% (HR for risk of death, 0.75 [95% CI, 0.47-1.19]; P = .22), and the estimated 5-year locoregional recurrence-free survival was 85.0% vs 80.8% (HR for risk of locoregional recurrence or death, 0.72 [95% CI, 0.46-1.13]; P = .15). The most common capecitabine-related adverse event was hand-foot syndrome (45.2%), with 7.7% of patients experiencing a grade 3 event.
Conclusions and relevance: Among women with early-stage triple-negative breast cancer who received standard adjuvant treatment, low-dose capecitabine maintenance therapy for 1 year, compared with observation, resulted in significantly improved 5-year disease-free survival.
Trial registration: ClinicalTrials.gov Identifier: NCT01112826.
Conflict of interest statement
Figures

Comment in
-
Adjuvant Capecitabine in Triple-Negative Breast Cancer: New Strategies for Tailoring Treatment Recommendations.JAMA. 2021 Jan 5;325(1):36-38. doi: 10.1001/jama.2020.23371. JAMA. 2021. PMID: 33300953 No abstract available.
-
Effect of Adjuvant Metronomic Capecitabine on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer.JAMA. 2021 May 4;325(17):1792-1793. doi: 10.1001/jama.2021.2783. JAMA. 2021. PMID: 33944880 No abstract available.
-
Effect of Adjuvant Metronomic Capecitabine on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer.JAMA. 2021 May 4;325(17):1791-1792. doi: 10.1001/jama.2021.2780. JAMA. 2021. PMID: 33944881 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

