The effect of differential force system and minimal surgical intervention on orthodontic tooth movement and root resorption
- PMID: 33300988
- PMCID: PMC8643401
- DOI: 10.1093/ejo/cjaa065
The effect of differential force system and minimal surgical intervention on orthodontic tooth movement and root resorption
Abstract
Objective: The primary objective of this study was to quantify the orthodontic tooth movement (OTM) and orthodontically induced root resorption (OIRR) with differential force system in conjunction with minimal surgical insult.
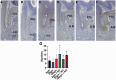
Material and methods: 15-week-old, 48 male Wistar rats were used in the research and were randomly divided into six groups: 1. Group 1 (8 Wistar rats): OTM for 14 days with 8-g force; 2. Group 2 (8 Wistar rats): OTM for 14 days with 25-g force; 3. Group 3 (8 Wistar rats): OTM for 14 days with 100-g force; 4. Group 4 (8 Wistar rats): OTM for 14 days with 8-g force and alveolar decortications (ADs); 5. Group 5 (8 Wistar rats): OTM for 14 days with 25-g force and ADs; 6. Group 6 (8 Wistar rats): OTM for 14 days with 100-g force and ADs. A nickel-titanium spring was used to protract the molar mesially using maxillary incisors as an anchorage. ADs (minimal surgical insult) were done using a hand piece and a round bur, adjacent to the left first maxillary molar on the palatal alveolar bone. After 14 days of OTM, Wistar rats were killed and microfocus computed tomography and histological analysis were performed.
Results: The 100-g group showed significant increase (P < 0.05) in OTM. However, with ADs, the OTM was significantly higher (P < 0.05) in 8 and 100 g. In addition, with ADs, there is significant increase (P < 0.05) in OIRR and significant decrease (P < 0.05) in bone volume fraction. Histological quantification of tartrate-resistant acid phosphatase indicated a significant increase (P < 0.05) in the number of osteoclasts with ADs when compared without ADs.
Conclusions: Light force in conjunction with ADs are optimal to accelerate the OTM. Additionally, ADs increases the OIRR.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Orthodontic Society. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures







References
-
- Masella, R.S. and Meister, M. (2006) Current concepts in the biology of orthodontic tooth movement. American Journal of Orthodontics and Dentofacial Orthopedics, 129, 458–468. - PubMed
-
- Huang, H., Williams, R.C., Kyrkanides, S.(2014) Accelerated orthodontic tooth movement: molecular mechanisms. American Journal of Orthodontics and Dentofacial Orthopedics, 146, 620–632. - PubMed
-
- von Böhl, M. and Kuijpers-Jagtman, A.M. (2009) Hyalinization during orthodontic tooth movement: a systematic review on tissue reactions. European Journal of Orthodontics, 31, 30–36. - PubMed

