Optimal Avapritinib Treatment Strategies for Patients with Metastatic or Unresectable Gastrointestinal Stromal Tumors
- PMID: 33301227
- PMCID: PMC8018323
- DOI: 10.1002/onco.13632
Optimal Avapritinib Treatment Strategies for Patients with Metastatic or Unresectable Gastrointestinal Stromal Tumors
Abstract
Background: Avapritinib, a novel inhibitor of KIT/PDGFRA, is approved in the U.S. for the treatment of adults with PDGFRA exon 18-mutant unresectable or metastatic gastrointestinal stromal tumors (U/M GISTs). We assessed the safety of avapritinib and provide evidence-based guidance on management of avapritinib-associated adverse events (AEs), including cognitive effects and intracranial bleeding.
Materials and methods: We performed a post hoc analysis of data from a two-part, single-arm dose escalation/expansion phase I study (NAVIGATOR; NCT02508532) in patients with U/M GISTs treated with oral avapritinib 30-600 mg once daily. The primary endpoints were safety and tolerability; the impact of dose modification (interruption and/or reduction) on progression-free survival (PFS) was a secondary endpoint. Efficacy analyses were limited to patients who started avapritinib at 300 mg (approved dose).
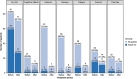
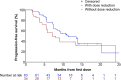
Results: Of 250 patients enrolled in the study, 74.0% presented with KIT mutation and 24.8% presented with PDGFRA exon 18-mutation; 66.8% started avapritinib at 300 mg. The most common treatment-related AEs (any grade) were nausea (59.2%), fatigue (50.0%), periorbital edema (42.0%), anemia (39.2%), diarrhea (36.0%), vomiting (36.0%), and increased lacrimation (30.8%). No treatment-related deaths occurred. Among 167 patients starting on 300 mg avapritinib, all-cause cognitive effects rate (grade 1-2) was 37.0% in all patients and 52.0% in patients ≥65 years. Cognitive effects improved to a lower grade more quickly with dose modification (1.3-3.1 weeks) than without (4.9-7.6 weeks). Median PFS was 11.4 months with dose modification and 7.2 months without.
Conclusion: Tolerability-guided dose modification of avapritinib is an effective strategy for managing AEs in patients with GISTs.
Implications for practice: Early recognition of adverse events and tailored dose modification appear to be effective approaches for managing treatment-related adverse events and maintaining patients on avapritinib. Dose reduction does not appear to result in reduced efficacy. Patients' cognitive function should be assessed at baseline and monitored carefully throughout treatment with avapritinib for the onset of cognitive adverse events. Dose interruption is recommended at the first sign of any cognitive effect, including grade 1 events.
Keywords: Avapritinib; Cognitive effects; Gastrointestinal stromal tumor; KIT; PDGFRA.
© 2020 The Authors. The Oncologist published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
Figures

References
-
- Soreide K, Sandvik OM, Soreide JA et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population‐based cohort studies. Cancer Epidemiol 2016;40:39–46. - PubMed
-
- Nilsson B, Bumming P, Meis‐Kindblom JM et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era–A population‐based study in western Sweden. Cancer 2005;103:821–829. - PubMed
-
- Casali PG, Abecassis N, Aro HT et al. Gastrointestinal stromal tumours: ESMO‐EURACAN clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2018;29:iv68–iv78. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

