Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- PMID: 33301246
- PMCID: PMC7745181
- DOI: 10.1056/NEJMoa2034577
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the resulting coronavirus disease 2019 (Covid-19) have afflicted tens of millions of people in a worldwide pandemic. Safe and effective vaccines are needed urgently.
Methods: In an ongoing multinational, placebo-controlled, observer-blinded, pivotal efficacy trial, we randomly assigned persons 16 years of age or older in a 1:1 ratio to receive two doses, 21 days apart, of either placebo or the BNT162b2 vaccine candidate (30 μg per dose). BNT162b2 is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein. The primary end points were efficacy of the vaccine against laboratory-confirmed Covid-19 and safety.
Results: A total of 43,548 participants underwent randomization, of whom 43,448 received injections: 21,720 with BNT162b2 and 21,728 with placebo. There were 8 cases of Covid-19 with onset at least 7 days after the second dose among participants assigned to receive BNT162b2 and 162 cases among those assigned to placebo; BNT162b2 was 95% effective in preventing Covid-19 (95% credible interval, 90.3 to 97.6). Similar vaccine efficacy (generally 90 to 100%) was observed across subgroups defined by age, sex, race, ethnicity, baseline body-mass index, and the presence of coexisting conditions. Among 10 cases of severe Covid-19 with onset after the first dose, 9 occurred in placebo recipients and 1 in a BNT162b2 recipient. The safety profile of BNT162b2 was characterized by short-term, mild-to-moderate pain at the injection site, fatigue, and headache. The incidence of serious adverse events was low and was similar in the vaccine and placebo groups.
Conclusions: A two-dose regimen of BNT162b2 conferred 95% protection against Covid-19 in persons 16 years of age or older. Safety over a median of 2 months was similar to that of other viral vaccines. (Funded by BioNTech and Pfizer; ClinicalTrials.gov number, NCT04368728.).
Copyright © 2020 Massachusetts Medical Society.
Figures
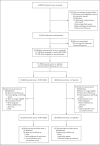
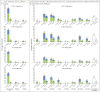
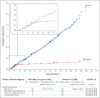

Comment in
-
SARS-CoV-2 Vaccination - An Ounce (Actually, Much Less) of Prevention.N Engl J Med. 2020 Dec 31;383(27):2677-2678. doi: 10.1056/NEJMe2034717. Epub 2020 Dec 10. N Engl J Med. 2020. PMID: 33301245 Free PMC article. No abstract available.
-
US authorization of first COVID vaccine marks new phase in safety monitoring.Nature. 2020 Dec;588(7838):377-378. doi: 10.1038/d41586-020-03542-4. Nature. 2020. PMID: 33311629 No abstract available.
-
[COVID-19 vaccine and anticoagulation patients at high cardiovascular risk. SEMERGEN recommendations].Semergen. 2021 Jan-Feb;47(1):1-3. doi: 10.1016/j.semerg.2020.12.005. Epub 2021 Jan 2. Semergen. 2021. PMID: 33478841 Free PMC article. Spanish. No abstract available.
-
Why two doses of the BNT162b2 mRNA COVID-19 vaccine? A different strategy to speed up the vaccination process: single-dose of the BNT162b2 mRNA COVID-19 and other vaccines.Panminerva Med. 2022 Mar;64(1):131-132. doi: 10.23736/S0031-0808.21.04286-5. Epub 2021 Jan 22. Panminerva Med. 2022. PMID: 33480519 No abstract available.
-
The BNT162b2 (BioNTech/Pfizer) vaccine had 95% efficacy against COVID-19 ≥7 days after the 2nd dose.Ann Intern Med. 2021 Feb;174(2):JC15. doi: 10.7326/ACPJ202102160-015. Epub 2021 Feb 2. Ann Intern Med. 2021. PMID: 33524290
-
COVID-19: Nobelpreiswürdiger Erfolg in der Impfstoff-Forschung.MMW Fortschr Med. 2021 Feb;163(2):24-25. doi: 10.1007/s15006-021-9577-4. MMW Fortschr Med. 2021. PMID: 33527275 Free PMC article. German. No abstract available.
-
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine.N Engl J Med. 2021 Apr 22;384(16):1576-1577. doi: 10.1056/NEJMc2036242. Epub 2021 Feb 17. N Engl J Med. 2021. PMID: 33596348 No abstract available.
-
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine.N Engl J Med. 2021 Apr 22;384(16):1577. doi: 10.1056/NEJMc2036242. Epub 2021 Feb 17. N Engl J Med. 2021. PMID: 33596349 No abstract available.
-
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine.N Engl J Med. 2021 Apr 22;384(16):1577-1578. doi: 10.1056/NEJMc2036242. Epub 2021 Feb 17. N Engl J Med. 2021. PMID: 33596350 No abstract available.
-
Safety and Considerations of the COVID-19 Vaccine Massive Deployment.Virol Sin. 2021 Oct;36(5):1097-1103. doi: 10.1007/s12250-021-00408-5. Epub 2021 Jun 1. Virol Sin. 2021. PMID: 34061319 Free PMC article. No abstract available.
-
Addressing Vaccine Inequity - Covid-19 Vaccines as a Global Public Good.N Engl J Med. 2022 Mar 24;386(12):1176-1179. doi: 10.1056/NEJMe2202547. Epub 2022 Feb 23. N Engl J Med. 2022. PMID: 35196425 No abstract available.
References
-
- Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2020 (https://coronavirus.jhu.edu/map.html).
-
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020 (https://www.who.int/director-general/speeches/detail/who-director-genera...).
-
- Centers for Disease Control and Prevention. COVID-19 information page (https://www.cdc.gov/coronavirus/2019-ncov/index.html).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
