Downregulation of epithelial DUOX1 in chronic obstructive pulmonary disease
- PMID: 33301419
- PMCID: PMC7934842
- DOI: 10.1172/jci.insight.142189
Downregulation of epithelial DUOX1 in chronic obstructive pulmonary disease
Abstract
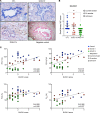
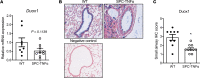
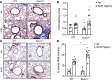
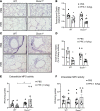
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease characterized by small airway remodeling and alveolar emphysema due to environmental stresses such as cigarette smoking (CS). Oxidative stress is commonly implicated in COPD pathology, but recent findings suggest that one oxidant-producing NADPH oxidase homolog, dual oxidase 1 (DUOX1), is downregulated in the airways of patients with COPD. We evaluated lung tissue sections from patients with COPD for small airway epithelial DUOX1 protein expression, in association with measures of lung function and small airway and alveolar remodeling. We also addressed the impact of DUOX1 for lung tissue remodeling in mouse models of COPD. Small airway DUOX1 levels were decreased in advanced COPD and correlated with loss of lung function and markers of emphysema and remodeling. Similarly, DUOX1 downregulation in correlation with extracellular matrix remodeling was observed in a genetic model of COPD, transgenic SPC-TNF-α mice. Finally, development of subepithelial airway fibrosis in mice due to exposure to the CS-component acrolein, or alveolar emphysema induced by administration of elastase, were in both cases exacerbated in Duox1-deficient mice. Collectively, our studies highlight that downregulation of DUOX1 may be a contributing feature of COPD pathogenesis, likely related to impaired DUOX1-mediated innate injury responses involved in epithelial homeostasis.
Keywords: COPD; Cell Biology; Extracellular matrix; Pulmonology.
Conflict of interest statement
Figures


References
-
- World Health Organization. Chronic obstructive pulmonary disease (COPD) (Fact Sheet March 2015). http://www.who.int/respiratory/copd/en/ Accessed December 4, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

