False-negative results of initial RT-PCR assays for COVID-19: A systematic review
- PMID: 33301459
- PMCID: PMC7728293
- DOI: 10.1371/journal.pone.0242958
False-negative results of initial RT-PCR assays for COVID-19: A systematic review
Abstract
Background: A false-negative case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is defined as a person with suspected infection and an initial negative result by reverse transcription-polymerase chain reaction (RT-PCR) test, with a positive result on a subsequent test. False-negative cases have important implications for isolation and risk of transmission of infected people and for the management of coronavirus disease 2019 (COVID-19). We aimed to review and critically appraise evidence about the rate of RT-PCR false-negatives at initial testing for COVID-19.
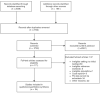
Methods: We searched MEDLINE, EMBASE, LILACS, as well as COVID-19 repositories, including the EPPI-Centre living systematic map of evidence about COVID-19 and the Coronavirus Open Access Project living evidence database. Two authors independently screened and selected studies according to the eligibility criteria and collected data from the included studies. The risk of bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. We calculated the proportion of false-negative test results using a multilevel mixed-effect logistic regression model. The certainty of the evidence about false-negative cases was rated using the GRADE approach for tests and strategies. All information in this article is current up to July 17, 2020.
Results: We included 34 studies enrolling 12,057 COVID-19 confirmed cases. All studies were affected by several risks of bias and applicability concerns. The pooled estimate of false-negative proportion was highly affected by unexplained heterogeneity (tau-squared = 1.39; 90% prediction interval from 0.02 to 0.54). The certainty of the evidence was judged as very low due to the risk of bias, indirectness, and inconsistency issues.
Conclusions: There is substantial and largely unexplained heterogeneity in the proportion of false-negative RT-PCR results. The collected evidence has several limitations, including risk of bias issues, high heterogeneity, and concerns about its applicability. Nonetheless, our findings reinforce the need for repeated testing in patients with suspicion of SARS-Cov-2 infection given that up to 54% of COVID-19 patients may have an initial false-negative RT-PCR (very low certainty of evidence).
Systematic review registration: Protocol available on the OSF website: https://tinyurl.com/vvbgqya.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19): Situation Report– 1. Geneve, Switzerland: 2020 January 20–2020. Report No.
-
- Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo IM, Halepoto DM, Iqbal M, et al. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. European review for medical and pharmacological sciences. 2020;24(4):2012–9. Epub 2020/03/07. 10.26355/eurrev_202002_20379 . - DOI - PubMed
-
- World Health Organization. Coronavirus disease 2019 (COVID-19): Situation Report– 51. Geneve, Switzerland: 2020 March 11–2020. Report No.
-
- World Health Organization. Weekly Operational Update on COVID-19: 16 October 2020. Geneve, Switzerland: 2020 October 16–2020. Report No.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

