ACAD10 protein expression and Neurobehavioral assessment of Acad10-deficient mice
- PMID: 33301490
- PMCID: PMC7728233
- DOI: 10.1371/journal.pone.0242445
ACAD10 protein expression and Neurobehavioral assessment of Acad10-deficient mice
Abstract
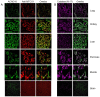
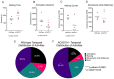
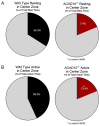
Acyl-CoA dehydrogenase 10 (Acad10)-deficient mice develop impaired glucose tolerance, peripheral insulin resistance, and abnormal weight gain. In addition, they exhibit biochemical features of deficiencies of fatty acid oxidation, such as accumulation of metabolites consistent with abnormal mitochondrial energy metabolism and fasting induced rhabdomyolysis. ACAD10 has significant expression in mouse brain, unlike other acyl-CoA dehydrogenases (ACADs) involved in fatty acid oxidation. The presence of ACAD10 in human tissues was determined using immunohistochemical staining. To characterize the effect of ACAD10 deficiency on the brain, micro-MRI and neurobehavioral evaluations were performed. Acad10-deficient mouse behavior was examined using open field testing and DigiGait analysis for changes in general activity as well as indices of gait, respectively. ACAD10 protein was shown to colocalize to mitochondria and peroxisomes in lung, muscle, kidney, and pancreas human tissue. Acad10-deficient mice demonstrated subtle behavioral abnormalities, which included reduced activity and increased time in the arena perimeter in the open field test. Mutant animals exhibited brake and propulsion metrics similar to those of control animals, which indicates normal balance, stability of gait, and the absence of significant motor impairment. The lack of evidence for motor impairment combined with avoidance of the center of an open field arena and reduced vertical and horizontal exploration are consistent with a phenotype characterized by elevated anxiety. These results implicate ACAD10 function in normal mouse behavior, which suggests a novel role for ACAD10 in brain metabolism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Lehninger, N., and Cox, Principles of Biochemistry, 2nd edition. 1982.
-
- Vockley, J., Bennett MJ, Gillingham MB Mitochondrial Fatty Acid Oxidation Disorders. in The Online Metabolic and Molecular Bases of Inherited Disease 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

