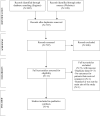
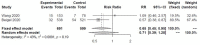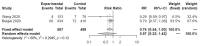Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis
- PMID: 33301514
- PMCID: PMC7728272
- DOI: 10.1371/journal.pone.0243705
Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis
Abstract
Background: Efficacy and safety of treatments for hospitalized COVID-19 are uncertain. We systematically reviewed efficacy and safety of remdesivir for the treatment of COVID-19.
Methods: Studies evaluating remdesivir in adults with hospitalized COVID-19 were searched in several engines until August 21, 2020. Primary outcomes included all-cause mortality, clinical improvement or recovery, need for invasive ventilation, and serious adverse events (SAEs). Inverse variance random effects meta-analyses were performed.
Results: We included four randomized controlled trials (RCTs) (n = 2296) [two vs. placebo (n = 1299) and two comparing 5-day vs. 10-day regimens (n = 997)], and two case series (n = 88). Studies used intravenous remdesivir 200mg the first day and 100mg for four or nine more days. One RCT (n = 236) was stopped early due to AEs; the other three RCTs reported outcomes between 11 and 15 days. Time to recovery was decreased by 4 days with remdesivir vs. placebo in one RCT (n = 1063), and by 0.8 days with 5-days vs. 10-days of therapy in another RCT (n = 397). Clinical improvement was better for 5-days regimen vs. standard of care in one RCT (n = 600). Remdesivir did not decrease all-cause mortality (RR 0.71, 95%CI 0.39 to 1.28, I2 = 43%) and need for invasive ventilation (RR 0.57, 95%CI 0.23 to 1.42, I2 = 60%) vs. placebo at 14 days but had fewer SAEs; 5-day decreased need for invasive ventilation and SAEs vs. 10-day in one RCT (n = 397). No differences in all-cause mortality or SAEs were seen among 5-day, 10-day and standard of care. There were some concerns of bias to high risk of bias in RCTs. Heterogeneity between studies could be due to different severities of disease, days of therapy before outcome determination, and how ordinal data was analyzed.
Conclusions: There is paucity of adequately powered and fully reported RCTs evaluating effects of remdesivir in hospitalized COVID-19 patients. Until stronger evidence emerges, we cannot conclude that remdesivir is efficacious for treating COVID-19.
Conflict of interest statement
The authors have read the journal’s policy and have the following potential competing interests: VP is a paid employee of MedErgy Health Group Inc., and PT is a paid employee of Hemex Health Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures
References
-
- Worldometer. COVID-19 Coronavirus Pandemic. [Accessed 8 May 2020] https://www.worldometers.info/coronavirus/.
-
- The OpenSAFELY Collaborative, Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, et al. OpenSAFELY: factors associated with COVID-19 related hospital deaths in the linked electronic health records of 17 million adult NHS patients. medRxiv [Pre-Print] 2020; May 7 10.1101/2020.05.06.20092999 - DOI
-
- World Health Organization. International Clinical Trials Registry Platform (ICTRP). https://www.who.int/ictrp/en/. Accessed May 8 2020.
-
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324(13):1307–1316. 10.1001/jama.2020.17021 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources