Navigating the Regulatory Pathways and Requirements for Tissue-Engineered Products in the Treatment of Burns in the United States
- PMID: 33301575
- PMCID: PMC8335953
- DOI: 10.1093/jbcr/iraa210
Navigating the Regulatory Pathways and Requirements for Tissue-Engineered Products in the Treatment of Burns in the United States
Abstract
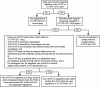
In the burn treatment landscape, a variety of skin substitutes, human tissue-sourced products, and other products are being developed based on tissue engineering (ie, the combination of scaffolds, cells, and biologically active molecules into functional tissue with the goal of restoring, maintaining, or improving damaged tissue or whole organs) to provide dermal replacement, prevent infection, or prevent or mitigate scarring. Skin substitutes can have a variety of compositions (cellular vs acellular), origins (human, animal, or synthetically derived), and complexities (dermal or epidermal only vs composite). The regulation of tissue-engineered products in the United States occurs by one of several pathways established by the U.S. Food and Drug Administration, including a Biologics License Application (BLA), a 510(k) (Class I and Class II devices), Premarket Approval (Class III devices), or a human cells, tissues, and cellular and tissue-based products designation. Key differentiators among these regulatory classifications include the amount and type of data required to support a filing. For example, a BLA requires a clinical trial(s) and evaluation of safety and efficacy by the Center for Biologics Evaluation and Research. Applicable approved biological products must also comply with submission of advertising and promotional materials per regulations. This review provides a description of, and associated requirements for, the various regulatory pathways for the approval or clearance of tissue-engineered products. Some of the regulatory challenges for commercialization of such products for the treatment of burns will be explored.
© The Author(s) 2020. Published by Oxford University Press on behalf of the American Burn Association.
Figures
References
-
- National Institute of Biomedical Imaging and Bioengineering. Tissue engineering and regenerative medicine. Accessed 2 June 2020; available from https://www.nibib.nih.gov/science-education/science-topics/tissue-engine....
-
- Castells-Sala C, Alemany-Ribes M, Fernández-Muiños T, et al. Current applications of tissue engineering in biomedicine. J Biochip Tissue Chip. 2013;S2:004. doi: 10.4172/2153-0777.S2-004. - DOI
-
- Snyder DL, Sullivan N, Schoelles KM.. Skin substitutes for treating chronic wounds. Technology Assessment Report. Rockville (MD): Agency for Healthcare Research and Quality (US);2012. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical