Treatment for chronic hepatitis E virus infection: A systematic review and meta-analysis
- PMID: 33301609
- PMCID: PMC7898834
- DOI: 10.1111/jvh.13456
Treatment for chronic hepatitis E virus infection: A systematic review and meta-analysis
Abstract
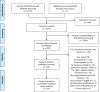
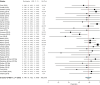
Hepatitis E virus infection can cause chronic hepatitis in immunocompromised patients with significant chance of progressive fibrosis and possibly cirrhosis. The aim of this systematic review was to summarize the efficacy and safety of the various treatment options for chronic hepatitis E. We performed a systematic literature search. The primary outcome measure was a sustained virological response (SVR). Secondary end points were rapid virological response (RVR), relapse rates, side effects and adverse events. Forty-four articles were included with a total of 582 patients. Reduction of immunosuppressive medication induced viral clearance in 55/174 (32%) of the patients. Meta-analysis of 395 patients showed a pooled SVR rate of 78% (95-CI 72%-84%) after ribavirin treatment. Twenty-five per cent of the patients obtained a RVR, whereas a relapse occurred in 18% of the patients. Anaemia during treatment led to dose reduction, use of erythropoietin and/or blood transfusion in 37% of the patients. A second treatment attempt with ribavirin led to a SVR in 39/51 (76%) of the patients. Pegylated interferon-alpha was administered to 13 patients and SVR was obtained in 85%. Two patients (15%) suffered from acute transplant rejection during treatment with interferon. In conclusion, reduction of immunosuppressive medication and treatment with ribavirin is safe, generally well tolerated and induced viral clearance in 32% and 78% of patients, respectively. Therefore, ribavirin should be considered as first treatment step for chronic hepatitis E. Treatment with pegylated interferon-alpha increases the risk of transplant rejection and should therefore be administered with great caution.
Keywords: hepatitis E virus; immunosuppression; ribavirin; systematic review.
© 2020 The Authors. Journal of Viral Hepatitis published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–97. - PubMed
-
- Ditah I, Ditah F, Devaki P, Ditah C, Kamath PS, Charlton M. Current epidemiology of hepatitis E virus infection in the United States: low seroprevalence in the National Health and Nutrition Evaluation Survey. Hepatology. 2014;60(3):815–22. - PubMed
-
- Stramer SL, Moritz ED, Foster GA, et al. Hepatitis E virus: seroprevalence and frequency of viral RNA detection among US blood donors. Transfusion. 2016;56(2):481–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources