One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab
- PMID: 33301613
- PMCID: PMC8246907
- DOI: 10.1111/ejh.13564
One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab
Abstract
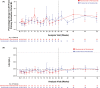
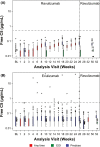
Ravulizumab every 8 weeks showed non-inferiority to eculizumab every 2 weeks in a 26-week, phase 3, randomized controlled trial in adults with paroxysmal nocturnal hemoglobinuria (PNH) who were clinically stable on eculizumab (NCT03056040). We report results from the first 26 weeks of the extension period in which patients continued ravulizumab (n = 96) or switched from eculizumab to ravulizumab (n = 95). At week 52, mean (SD) lactate dehydrogenase levels increased 8.8% (29%) with ravulizumab-ravulizumab and 5.8% (27%) with eculizumab-ravulizumab from primary evaluation period baseline. During the extension period, four patients (ravulizumab-ravulizumab, n = 3; eculizumab-ravulizumab, n = 1) experienced breakthrough hemolysis, but none associated with serum free C5 ≥ 0.5 μg/mL. Mean Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores remained stable through week 52. During the extension period, proportions of patients avoiding transfusion remained stable (ravulizumab-ravulizumab, 86.5%; eculizumab-ravulizumab, 83.2%); 81.2% and 81.1%, respectively, had stabilized hemoglobin. All patients maintained serum free C5 levels < 0.5 μg/mL. Adverse events were generally similar between groups, and rates were lower in the extension period. Adults with PNH on stable eculizumab therapy who received ravulizumab over 52 weeks experienced durable efficacy, with consistent efficacy in patients who received eculizumab during the primary evaluation period and then switched to ravulizumab. Ravulizumab was well tolerated.
Keywords: breakthrough hemolysis; complement inhibitor; hemoglobin; paroxysmal nocturnal hemoglobinuria; quality of life; transfusion.
© 2020 The Authors. European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures
References
-
- Devalet B, Mullier F, Chatelain B, Dogne JM, Chatelain C. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190‐198. - PubMed
-
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256‐1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

