Mitochondrial Ca2+, redox environment and ROS emission in heart failure: Two sides of the same coin?
- PMID: 33301801
- PMCID: PMC7880885
- DOI: 10.1016/j.yjmcc.2020.11.013
Mitochondrial Ca2+, redox environment and ROS emission in heart failure: Two sides of the same coin?
Abstract
Heart failure (HF) is a progressive, debilitating condition characterized, in part, by altered ionic equilibria, increased ROS production and impaired cellular energy metabolism, contributing to variable profiles of systolic and diastolic dysfunction with significant functional limitations and risk of premature death. We summarize current knowledge concerning changes of intracellular Na+ and Ca2+ control mechanisms during the disease progression and their consequences on mitochondrial Ca2+ homeostasis and the shift in redox balance. Absent existing biological data, our computational modeling studies advance a new 'in silico' analysis to reconcile existing opposing views, based on different experimental HF models, regarding variations in mitochondrial Ca2+ concentration that participate in triggering and perpetuating oxidative stress in the failing heart and their impact on cardiac energetics. In agreement with our hypothesis and the literature, model simulations demonstrate the possibility that the heart's redox status together with cytoplasmic Na+ concentrations act as regulators of mitochondrial Ca2+ levels in HF and of the bioenergetics response that will ultimately drive ATP supply and oxidative stress. The resulting model predictions propose future directions to study the evolution of HF as well as other types of heart disease, and to develop novel testable mechanistic hypotheses that may lead to improved therapeutics.
Keywords: Ca(2+) signaling; Computational modeling; Energetic adaptation; Intracellular sodium; Oxidative stress; Redox signaling.
Published by Elsevier Ltd.
Figures

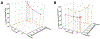
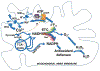
References
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, et al., 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, Circulation 128(16) (2013) 1810–52. - PubMed
-
- Dunlay SM, Roger VL, Redfield MM, Epidemiology of heart failure with preserved ejection fraction, Nat Rev Cardiol 14(10) (2017) 591–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

