Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: observational comparative study using routine care data
- PMID: 33301928
- PMCID: PMC7722522
- DOI: 10.1016/j.cmi.2020.11.035
Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: observational comparative study using routine care data
Abstract
Objective: To assess the effectiveness of corticosteroids on outcomes of patients with coronavirus disease 2019 (COVID-19) pneumonia requiring oxygen without mechanical ventilation.
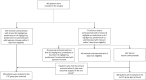
Methods: We used routine care data from 51 hospitals in France and Luxembourg to assess the effectiveness of corticosteroids at 0.8 mg/kg/day eq. prednisone (CTC group) versus standard of care (no-CTC group) among adults 18-80 years old with confirmed COVID-19 pneumonia requiring oxygen without mechanical ventilation. The primary outcome was intubation or death by day 28. In our main analysis, characteristics of patients at baseline (i.e. time when patients met all inclusion criteria) were balanced by using propensity-score inverse probability of treatment weighting.
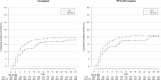
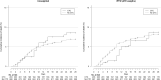
Results: Among the 891 patients included in the analysis, 203 were assigned to the CTC group. Use of corticosteroids was not significantly associated with risk of intubation or death by day 28 (weighted hazard ratio (wHR) 0.92, 95%CI 0.61-1.39) nor cumulative death rate (wHR 1.03, 95%CI 0.54-1.98). However, use of corticosteroids was associated with reduced risk of intubation or death by day 28 in the prespecified subgroups of patients requiring oxygen ≥3 L/min (wHR 0.50, 95%CI 0.30-0.85) or C-reactive protein level ≥100 mg/L (wHR 0.44, 95%CI 0.23-0.85). The number of hyperglycaemia events was higher for patients with corticosteroids than for those without, but the number of infections was similar.
Conclusions: We found no association between the use of corticosteroids and intubation or death in the broad population of patients 18-80 years old, with COVID-19, hospitalized in settings non intensive care units. However, the treatment was associated with a reduced risk of intubation or death for patients with ≥3 L/min oxygen or C-reactive protein level ≥100 mg/L at baseline. Further research is needed to confirm the right timing for corticosteroids in patients with COVID-19 requiring oxygen only.
Keywords: COVID-19; Causal inference; Corticosteroids; Observational study; Therapeutic evaluation.
Copyright © 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

