COVID-19 and Hyperimmune sera: A feasible plan B to fight against coronavirus
- PMID: 33302034
- PMCID: PMC7678452
- DOI: 10.1016/j.intimp.2020.107220
COVID-19 and Hyperimmune sera: A feasible plan B to fight against coronavirus
Abstract
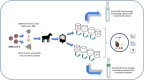
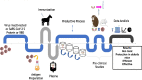
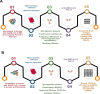
Since the very beginning of the COVID-19 pandemic, different treatment strategies have been explored. These mainly involve the development of antimicrobial, antiviral, and/or anti-inflammatory agents as well as vaccine production. However, other potential options should be more avidly investigated since vaccine production on a worldwide level, and the anti-vaccination movement, also known as anti-vax or vaccine hesitancy by many communities, are still real obstacles without a ready solution. This review presents recent findings on the potential therapeutic advantages of heterologous serotherapy for the treatment of COVID-19. We present not only the effective use in animal models of hyperimmune sera against this coronavirus but also strategies, and protocols for the production of anti-SARS-CoV-2 sera. Promising antigens are also indicated such as the receptor-binding domain (RBD) in SARS-CoV-2 S protein, which is already in phase 2/3 clinical trial, and the trimeric protein S, which was shown to be up to 150 times more potent than the serum from convalescent donors. Due to the high death rate, the treatment for those currently infected with coronavirus cannot be ignored. Therefore, the potential use of anti-SARS-CoV-2 hyperimmune sera should be carefully but urgently evaluated in phase 2/3 clinical studies.
Keywords: Animal antibodies; COVID-19; Coronavirus; Hyperimmune sera; Neutralizing titers; SARS-COV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
SARS-CoV-2 Neutralization in Convalescent Plasma and Commercial Lots of Plasma-Derived Immunoglobulin.BioDrugs. 2022 Jan;36(1):41-53. doi: 10.1007/s40259-021-00511-9. Epub 2021 Nov 29. BioDrugs. 2022. PMID: 34843105 Free PMC article.
-
Structural Basis of a Human Neutralizing Antibody Specific to the SARS-CoV-2 Spike Protein Receptor-Binding Domain.Microbiol Spectr. 2021 Oct 31;9(2):e0135221. doi: 10.1128/Spectrum.01352-21. Epub 2021 Oct 13. Microbiol Spectr. 2021. PMID: 34643438 Free PMC article.
-
Potent Anti-SARS-CoV-2 Efficacy of COVID-19 Hyperimmune Globulin from Vaccine-Immunized Plasma.Adv Sci (Weinh). 2022 May;9(14):e2104333. doi: 10.1002/advs.202104333. Epub 2022 Apr 11. Adv Sci (Weinh). 2022. PMID: 35403837 Free PMC article.
-
Anti-SARS-CoV-2 hyperimmune plasma workflow.Transfus Apher Sci. 2020 Oct;59(5):102850. doi: 10.1016/j.transci.2020.102850. Epub 2020 Jun 10. Transfus Apher Sci. 2020. PMID: 32540345 Free PMC article. Review.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 20;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Feb 1;2:CD013600. doi: 10.1002/14651858.CD013600.pub5. PMID: 34013969 Free PMC article. Updated.
Cited by
-
Non-clinical safety assessment and in vivo biodistribution of CoviFab, an RBD-specific F(ab')2 fragment derived from equine polyclonal antibodies.Toxicol Appl Pharmacol. 2022 Jan 1;434:115796. doi: 10.1016/j.taap.2021.115796. Epub 2021 Nov 14. Toxicol Appl Pharmacol. 2022. PMID: 34785274 Free PMC article.
-
Using in vivo animal models for studying SARS-CoV-2.Expert Opin Drug Discov. 2022 Feb;17(2):121-137. doi: 10.1080/17460441.2022.1995352. Epub 2021 Nov 3. Expert Opin Drug Discov. 2022. PMID: 34727803 Free PMC article.
-
Assessment of health impacts in retired antisera-producing horses: Blood biochemistry and serum amyloid A analysis.Vet World. 2024 Sep;17(9):2136-2143. doi: 10.14202/vetworld.2024.2136-2143. Epub 2024 Sep 20. Vet World. 2024. PMID: 39507779 Free PMC article.
-
Immune Maturation Effects on Viral Neutralization and Avidity of Hyperimmunized Equine Anti-SARS-CoV-2 Sera.Antibodies (Basel). 2022 Jan 2;11(1):3. doi: 10.3390/antib11010003. Antibodies (Basel). 2022. PMID: 35076465 Free PMC article.
-
Anti-SARS-CoV-2 and anti-cytokine storm neutralizing antibody therapies against COVID-19: Update, challenges, and perspectives.Int Immunopharmacol. 2021 Oct;99:108036. doi: 10.1016/j.intimp.2021.108036. Epub 2021 Aug 3. Int Immunopharmacol. 2021. PMID: 34371330 Free PMC article. Review.
References
-
- Zhou Q.A., Kato-Weinstein J., Li Y., Deng Y.i., Granet R., Garner L., Liu C., Polshakov D., Gessner C., Watkins S. Potential therapeutic agents and associated bioassay data for COVID-19 and related human coronavirus infections. ACS Pharmacol. Transl. Sci. 2020;3(5):813–834. doi: 10.1021/acsptsci.0c00074.s001. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

