COVID-19 and Hyperimmune sera: A feasible plan B to fight against coronavirus
- PMID: 33302034
- PMCID: PMC7678452
- DOI: 10.1016/j.intimp.2020.107220
COVID-19 and Hyperimmune sera: A feasible plan B to fight against coronavirus
Abstract
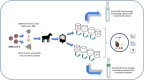
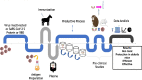
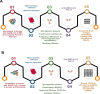
Since the very beginning of the COVID-19 pandemic, different treatment strategies have been explored. These mainly involve the development of antimicrobial, antiviral, and/or anti-inflammatory agents as well as vaccine production. However, other potential options should be more avidly investigated since vaccine production on a worldwide level, and the anti-vaccination movement, also known as anti-vax or vaccine hesitancy by many communities, are still real obstacles without a ready solution. This review presents recent findings on the potential therapeutic advantages of heterologous serotherapy for the treatment of COVID-19. We present not only the effective use in animal models of hyperimmune sera against this coronavirus but also strategies, and protocols for the production of anti-SARS-CoV-2 sera. Promising antigens are also indicated such as the receptor-binding domain (RBD) in SARS-CoV-2 S protein, which is already in phase 2/3 clinical trial, and the trimeric protein S, which was shown to be up to 150 times more potent than the serum from convalescent donors. Due to the high death rate, the treatment for those currently infected with coronavirus cannot be ignored. Therefore, the potential use of anti-SARS-CoV-2 hyperimmune sera should be carefully but urgently evaluated in phase 2/3 clinical studies.
Keywords: Animal antibodies; COVID-19; Coronavirus; Hyperimmune sera; Neutralizing titers; SARS-COV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Zhou Q.A., Kato-Weinstein J., Li Y., Deng Y.i., Granet R., Garner L., Liu C., Polshakov D., Gessner C., Watkins S. Potential therapeutic agents and associated bioassay data for COVID-19 and related human coronavirus infections. ACS Pharmacol. Transl. Sci. 2020;3(5):813–834. doi: 10.1021/acsptsci.0c00074.s001. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

