Metabolic Dysregulation and Neurovascular Dysfunction in Diabetic Retinopathy
- PMID: 33302369
- PMCID: PMC7762582
- DOI: 10.3390/antiox9121244
Metabolic Dysregulation and Neurovascular Dysfunction in Diabetic Retinopathy
Abstract
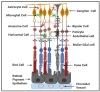
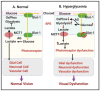
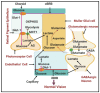
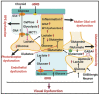
Diabetic retinopathy is a major cause of ocular complications in patients with type 1 and type 2 diabetes in developed countries. Due to the continued increase in the number of people with obesity and diabetes in the United States of America and globally, the incidence of diabetic retinopathy is expected to increase significantly in the coming years. Diabetic retinopathy is widely accepted as a combination of neurodegenerative and microvascular changes; however, which change occurs first is not yet understood. Although the pathogenesis of diabetic retinopathy is very complex, regulated by numerous signaling pathways and cellular processes, maintaining glucose homeostasis is still an essential component for normal physiological functioning of retinal cells. The maintenance of glucose homeostasis is finely regulated by coordinated interplay between glycolysis, Krebs cycle, and oxidative phosphorylation. Glycolysis is the most conserved metabolic pathway in biology and is tightly regulated to maintain a steady-state concentration of glycolytic intermediates; this regulation is called scheduled or regulated glycolysis. However, an abnormal increase in glycolytic flux generates large amounts of intermediate metabolites that can be shunted into different damaging pathways including the polyol pathway, hexosamine pathway, diacylglycerol-dependent activation of the protein kinase C pathway, and Amadori/advanced glycation end products (AGEs) pathway. In addition, disrupting the balance between glycolysis and oxidative phosphorylation leads to other biochemical and molecular changes observed in diabetic retinopathy including endoplasmic reticulum-mitochondria miscommunication and mitophagy dysregulation. This review will focus on how dysregulation of glycolysis contributes to diabetic retinopathy.
Keywords: diabetic retinopathy; endothelial cell; glycolytic overload; glycolytic pathway; hyperglycemia; metabolic deregulation; neurovascular dysfunction.
Conflict of interest statement
The authors declare no conflict of interest
Figures

References
-
- Sivaprasad S., Gupta B., Gulliford M.C., Dodhia H., Mohamed M., Nagi D., Evans J.R. Ethnic variations in the prevalence of diabetic retinopathy in people with diabetes attending screening in the United Kingdom (DRIVE UK) PLoS ONE. 2012;7:e32182. doi: 10.1371/journal.pone.0032182. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

