Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis
- PMID: 33302372
- PMCID: PMC7762540
- DOI: 10.3390/jof6040347
Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis
Abstract
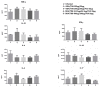
The peptide P10 is a vaccine candidate for Paracoccidioidomycosis, a systemic mycosis caused by fungal species of the genus Paracoccidioides spp. We have previously shown that peptide P10 vaccination, in the presence of several different adjuvants, induced a protective cellular immune response mediated by CD4+ Th1 lymphocytes that was associated with the increased production of IFN-γ in mice challenged with a virulent isolate of Paracoccidoides brasiliensis. Cationic liposomes formulated with dioctadecyldimethylammonium and trehalose dibehenate (DDA/TDB, termed also CAF01-cationic adjuvant formulation) have been developed for safe administration in humans and CAF01 liposomes are utilized as an adjuvant for modulating a robust Th1/Th17 cellular response. We evaluated the efficacy of the adsorption of peptide P10 to CAF01 cationic liposomes and used the generated liposomes to vaccinate C57Bl/6 mice infected with P. brasiliensis. Our results showed that P10 was efficiently adsorbed onto CAF01 liposomes. The vaccination of infected mice with cationic liposomes formulated with DDA/TDB 250/50 µg/mL and 20 µg of P10 induced an effective cellular immune response with increased levels of Th17 cytokines, which correlated with significant decreases in the fungal burdens in lungs and protective granulomatous tissue responses. Hence, cationic liposomes of DDA/TDB 250/50 µg/mL with 20 µg of P10 are a promising therapeutic for safely and effectively improving the treatment of paracoccidioidomycosis.
Keywords: CAF01; DDA/TDB; P. brasiliensis; adjuvant; cationic liposome; paracoccidioidomycosis; peptide vaccine.
Conflict of interest statement
There are no conflict of interest to declare.
Figures



References
-
- Souza A.C.O., Taborda C.P. Reference Module in Life Sciences. Elsevier BV; Amsterdam, The Netherlands: 2020. Epidemiology of Dimorphic Fungi.
-
- Shikanai-Yasuda M.A., Mendes P.R., Colombo A.L., De Queiroz-Telles F., Kono A.S.G., Paniago A.M.M., Nathan A., Valle A.C.F.D., Bagagli E., Benard G., et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017;50:715–740. doi: 10.1590/0037-8682-0230-2017. - DOI - PubMed
-
- Matute D.R., McEwen J.G., Puccia R., Montes B.A., San-Blas G., Bagagli E., Rauscher J.T., Restrepo A., Morais F., Nino-Vega G., et al. Cryptic Speciation and Recombination in the Fungus Paracoccidioides brasiliensis as Revealed by Gene Genealogies. Mol. Biol. Evol. 2006;23:65–73. doi: 10.1093/molbev/msj008. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

