Exercise and Nutrition for Healthy AgeiNg (ENHANce) project - effects and mechanisms of action of combined anabolic interventions to improve physical functioning in sarcopenic older adults: study protocol of a triple blinded, randomized controlled trial
- PMID: 33302879
- PMCID: PMC7727134
- DOI: 10.1186/s12877-020-01900-5
Exercise and Nutrition for Healthy AgeiNg (ENHANce) project - effects and mechanisms of action of combined anabolic interventions to improve physical functioning in sarcopenic older adults: study protocol of a triple blinded, randomized controlled trial
Abstract
Background: The Exercise and Nutrition for Healthy AgeiNg (ENHANce) project aims to assess the combined effects of exercise and nutritional interventions to prevent loss of skeletal muscle mass and function with ageing, and to determine the underlying mechanisms of action.
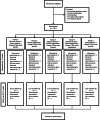
Methods: One hundred eightycommunity-dwelling sarcopenic individuals (≥ 65 years) are allocated in a randomized controlled trial (RCT) in a 1:1 ratio into five groups for a 12-week intervention period, followed by a 12-week follow-up period: 1) exercise intervention +protein placebo +omega-3 fatty acids placebo; 2) protein +omega-3 fatty acids placebo; 3) exercise intervention +protein +omega-3 fatty acids placebo; 4) exercise intervention +protein +omega-3 fatty acids; 5) protein placebo +omega-3 fatty acids placebo. All interventions are in line with recommendations of expert groups such as the American College of Sports Medicine and the PROT-AGE study group and individualized to the physical capabilities and nutritional intake of each participant. Sarcopenia is diagnosed by the assessment of gait speed, handgrip strength (Jamar handheld dynamometer), chair stand test and muscle mass (DXA) according to the European Working Group on Sarcopenia in Older People (EWGSOP2) criteria. Participants, researchers and statisticians are blinded to omega-3 fatty acids and protein treatment. Compliance to the exercise program, protein and omega-3 fatty acids interventions is objectively measured, by monitoring movement by an activity monitor, determining nitrogen content in urine and analyzing the fatty acid composition of the red blood cell membrane. The primary outcome of the RCT is the change in Short Physical Performance Battery (SPPB) score. Secondary endpoints are, among others, changes in muscle mass, strength and function, objective compliance to interventions, changes in muscle and blood biomarkers related to sarcopenia, cognition, quality of life and falls.
Discussion: This RCT in well-defined sarcopenic older adults assesses the effects of combined anabolic interventions, including the additive effects of omega-3 fatty acids supplements, compared to single or placebo interventions. Compliance with the exercise intervention and with the intake of nutritional supplements is measured objectively. Also, blood and muscle samples will be used to explore the underlying determinants that contribute to the mechanism of action of anabolic interventions.
Trial registration: Clinicaltrials.gov: NCT03649698 , retrospectively registered at 28 August 2018, first participant was randomized 16 February 2018.
Keywords: Community-dwelling; Compliance; Exercise intervention; Frailty; Older adults; Omega-3 fatty acids; Protein supplementation; Randomized controlled trial; Sarcopenia.
Conflict of interest statement
None declared. This research was commercially funded by Vista-life® (discounted rate for the products Vista®-omega-3 and placebo product) and Nestlé® Health Science Belgium (discounted rate for the products Resource® Instant Protein and Resource® Maltodextrin). Neither Vista-life®, nor Nestlé® Health Science Belgium contributed to or influenced the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
References
-
- Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56. doi: 10.1093/ageing/afy106. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical