Increased Association of Deamidated αA-N101D with Lens membrane of transgenic αAN101D vs. wild type αA mice: potential effects on intracellular ionic imbalance and membrane disorganization
- PMID: 33302904
- PMCID: PMC7726915
- DOI: 10.1186/s12886-020-01734-0
Increased Association of Deamidated αA-N101D with Lens membrane of transgenic αAN101D vs. wild type αA mice: potential effects on intracellular ionic imbalance and membrane disorganization
Abstract
We have generated two mouse models, in one by inserting the human lens αAN101D transgene in CRYαAN101D mice, and in the other by inserting human wild-type αA-transgene in CRYαAWT mice. The CRYαAN101D mice developed cortical cataract at about 7-months of age relative to CRYαAWT mice. The objective of the study was to determine the following relative changes in the lenses of CRYαAN101D- vs. CRYαAWT mice: age-related changes with specific emphasis on protein insolubilization, relative membrane-association of αAN101D vs. WTαA proteins, and changes in intracellular ionic imbalance and membrane organization.
Methods: Lenses of varying ages from CRYαAWT and CRYαAN101D mice were compared for an age-related protein insolubilization. The relative lens membrane-association of the αAN101D- and WTαA proteins in the two types of mice was determined by immunohistochemical-, immunogold-labeling-, and western blot analyses. The relative levels of membrane-binding of recombinant αAN101D- and WTαA proteins was determined by an in vitro assay, and the levels of intracellular Ca2+ uptake and Na, K-ATPase mRNA were determined in the cultured epithelial cells from lenses of the two types of mice.
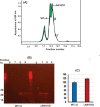
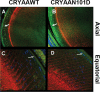
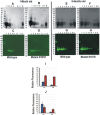
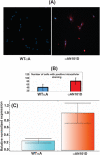
Results: Compared to the lenses of CRYαAWT, the lenses of CRYαAN101D mice exhibited: (A) An increase in age-related protein insolubilization beginning at about 4-months of age. (B) A greater lens membrane-association of αAN101D- relative to WTαA protein during immunogold-labeling- and western blot analyses, including relatively a greater membrane swelling in the CRYαAN101D lenses. (C) During in vitro assay, the greater levels of binding αAN101D- relative to WTαA protein to membranes was observed. (D) The 75% lower level of Na, K-ATPase mRNA but 1.5X greater Ca2+ uptake were observed in cultured lens epithelial cells of CRYαAN101D- than those of CRYαAWT mice.
Conclusions: The results show that an increased lens membrane association of αAN101D--relative WTαA protein in CRYαAN101D mice than CRYαAWT mice occurs, which causes intracellular ionic imbalance, and in turn, membrane swelling that potentially leads to cortical opacity.
Keywords: Cataract; Crystallins; Deamidation; Lens; Post-translational modifications; Transgenic mice.
Conflict of interest statement
No competing interest.
Figures




Similar articles
-
Measurement of absolute abundance of crystallins in human and αA N101D transgenic mouse lenses using 15N-labeled crystallin standards.Exp Eye Res. 2024 Nov;248:110115. doi: 10.1016/j.exer.2024.110115. Epub 2024 Oct 3. Exp Eye Res. 2024. PMID: 39368693
-
The common modification in alphaA-crystallin in the lens, N101D, is associated with increased opacity in a mouse model.J Biol Chem. 2011 Apr 1;286(13):11579-92. doi: 10.1074/jbc.M110.148627. Epub 2011 Jan 18. J Biol Chem. 2011. PMID: 21245144 Free PMC article.
-
Molecular mechanism of formation of cortical opacity in CRYAAN101D transgenic mice.Invest Ophthalmol Vis Sci. 2014 Aug 21;55(10):6398-408. doi: 10.1167/iovs.14-14623. Invest Ophthalmol Vis Sci. 2014. PMID: 25146988 Free PMC article.
-
A transgenic mouse model for human autosomal dominant cataract.Invest Ophthalmol Vis Sci. 2006 May;47(5):2036-44. doi: 10.1167/iovs.05-0524. Invest Ophthalmol Vis Sci. 2006. PMID: 16639013 Free PMC article.
-
Lens β-crystallins: the role of deamidation and related modifications in aging and cataract.Prog Biophys Mol Biol. 2014 Jul;115(1):21-31. doi: 10.1016/j.pbiomolbio.2014.02.004. Epub 2014 Mar 6. Prog Biophys Mol Biol. 2014. PMID: 24613629 Free PMC article. Review.
Cited by
-
Measurement of absolute abundance of crystallins in human and αA N101D transgenic mouse lenses using 15N-labeled crystallin standards.Exp Eye Res. 2024 Nov;248:110115. doi: 10.1016/j.exer.2024.110115. Epub 2024 Oct 3. Exp Eye Res. 2024. PMID: 39368693
-
Alpha-Crystallin-Membrane Association Modulated by Phospholipid Acyl Chain Length and Degree of Unsaturation.Membranes (Basel). 2022 Apr 23;12(5):455. doi: 10.3390/membranes12050455. Membranes (Basel). 2022. PMID: 35629781 Free PMC article.
-
An AFM Approach Applied in a Study of α-Crystallin Membrane Association: New Insights into Lens Hardening and Presbyopia Development.Membranes (Basel). 2022 May 14;12(5):522. doi: 10.3390/membranes12050522. Membranes (Basel). 2022. PMID: 35629848 Free PMC article.
-
G91-deletion in βA3/A1-crystallin induces cellular and molecular changes in mouse lenses leading to congenital cataract development.PLoS One. 2025 Jul 7;20(7):e0326305. doi: 10.1371/journal.pone.0326305. eCollection 2025. PLoS One. 2025. PMID: 40622998 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

