Outcomes and safety of concomitant topiramate or metformin for antipsychotics-induced obesity: a randomized-controlled trial
- PMID: 33302986
- PMCID: PMC7727176
- DOI: 10.1186/s12991-020-00319-x
Outcomes and safety of concomitant topiramate or metformin for antipsychotics-induced obesity: a randomized-controlled trial
Abstract
Background: Although there are some existing data describing the usage of topiramate in patients with antipsychotic-induced obesity, study on its comparison with metformin is limited. This study aimed to explore the effectiveness and safety of concomitant topiramate on antipsychotic-induced obesity as well as its comparison with metformin.
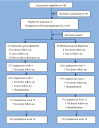
Methods: 62 stabilized outpatients with antipsychotic-induced obesity were randomized into the topiramate group and the metformin group with 16-week treatment. The patients' weight, body mass index (BMI), waist-hip ratio, and their side effects were assessed and compared. Intention-to-treat and completer analyses were performed. Meanwhile, covariance analysis was conducted to control the impact of the significant difference in BMI between the two groups.
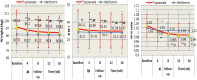
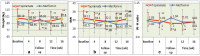
Results: The two groups had comparable characteristics, though their difference in baseline BMI was significant. (1) Intention-to-treat analyses: the random missing values were replaced using the last observation carried forward method when intention-to-treat analyses were conducted. Compared with the baseline, the weight, BMI, and waist-hip ratio in the topiramate group markedly decreased at each follow-up, whereas, in the metformin group, only waist-hip ratio significantly decreased at 4 weeks after treatment. Compared with the metformin, only weight and BMI in the topiramate group were significantly decreased at week 4 after treatment, and at week 8-16, weight, BMI and waist-hip ratio were remarkably declined. (2) Completer analyses: compared with the baseline, the weight, BMI, and waist-hip ratio in the topiramate group at week 4-16 were markedly decreased, whereas only waist-hip ratio with metformin was significantly decreased at week 4. Compared with the metformin, all BMI with topiramate were markedly decreased at week 4-16. Moreover, its weight and waist-hip ratio also were notably lowered at week 8. No significant differences in adverse events were found between the two groups.
Conclusions: Topiramate, similar to metformin in reducing obesity as previously reported, also significantly reduced body weight, BMI, and waist-hip ratio in patients with antipsychotic-induced obesity and demonstrated well tolerance in psychiatric patients. Trial registration The trial was registered at http://www.chictr.org.cn , and the number was ChiCTR-IPR-17013122.
Keywords: Antipsychotics; Metformin; Obesity; Topiramate.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors declare that they have no competing interests.
Figures
References
-
- Anagnostou E, Aman MG, Handen BL, Sanders KB, Shui A, Hollway JA, et al. Metformin for treatment of overweight induced by atypical antipsychotic medication in young people with autism spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):928–937. doi: 10.1001/jamapsychiatry.2016.1232. - DOI - PubMed
-
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. - DOI - PMC - PubMed
-
- Erickson ZD, Kwan CL, Gelberg HA, Arnold IY, Chamberlin V, Rosen JA, et al. A Randomized, controlled multisite study of behavioral interventions for veterans with mental illness and antipsychotic medication-associated obesity. J Gen Intern Med. 2017;32(Suppl 1):32–39. doi: 10.1007/s11606-016-3960-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

