Rescue of multiple class II CFTR mutations by elexacaftor+tezacaftor+ivacaftor mediated in part by the dual activities of elexacaftor as both corrector and potentiator
- PMID: 33303536
- PMCID: PMC8209484
- DOI: 10.1183/13993003.02774-2020
Rescue of multiple class II CFTR mutations by elexacaftor+tezacaftor+ivacaftor mediated in part by the dual activities of elexacaftor as both corrector and potentiator
Abstract
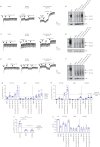
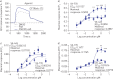
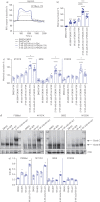
Positive results in pre-clinical studies of the triple combination of elexacaftor, tezacaftor and ivacaftor, performed in airway epithelial cell cultures obtained from patients harbouring the class II cystic fibrosis transmembrane conductance regulator (CFTR) mutation F508del-CFTR, translated to impressive clinical outcomes for subjects carrying this mutation in clinical trials and approval of Trikafta.Encouraged by this correlation, we were prompted to evaluate the effect of the elexacaftor, tezacaftor and ivacaftor triple combination on primary nasal epithelial cultures obtained from individuals with rare class II CF-causing mutations (G85E, M1101K and N1303K) for which Trikafta is not approved.Cultures from individuals homozygous for M1101K responded better than cultures harbouring G85E and N1303K after treatment with the triple combination with respect to improvement in regulated channel function and protein processing. A similar genotype-specific effect of the triple combination was observed when the different mutations were expressed in HEK293 cells, supporting the hypothesis that these modulators may act directly on the mutant proteins. Detailed studies in nasal cultures and HEK293 cells showed that the corrector, elexacaftor, exhibited dual activity as both corrector and potentiator, and suggested that the potentiator activity contributes to its pharmacological activity.These pre-clinical studies using nasal epithelial cultures identified mutation genotypes for which elexacaftor, tezacaftor and ivacaftor may produce clinical responses that are comparable to, or inferior to, those observed for F508del-CFTR.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: O. Laselva has nothing to disclose. Conflict of interest: C. Bartlett has nothing to disclose. Conflict of interest: T.N.A. Gunawardena has nothing to disclose. Conflict of interest: H. Ouyang has nothing to disclose. Conflict of interest: P.D.W. Eckford has nothing to disclose. Conflict of interest: T.J. Moraes has nothing to disclose. Conflict of interest: C.E. Bear reports grants from CF Canada and Canadian Institutes of Health, during the conduct of the study; a philanthropic donation to the author's host institution from Jagle Bash, outside the submitted work. Conflict of interest: T. Gonska reports grants from CF Canada/SickKids Foundation, during the conduct of the study; travel reimbursement for lectures and for participation on an advisory board from Vertex Pharmaceutical Inc., outside the submitted work.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
