Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial
- PMID: 33303539
- PMCID: PMC7736754
- DOI: 10.1183/13993003.03481-2020
Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial
Abstract
Background: After the 2002/2003 severe acute respiratory syndrome outbreak, 30% of survivors exhibited persisting structural pulmonary abnormalities. The long-term pulmonary sequelae of coronavirus disease 2019 (COVID-19) are yet unknown, and comprehensive clinical follow-up data are lacking.
Methods: In this prospective, multicentre, observational study, we systematically evaluated the cardiopulmonary damage in subjects recovering from COVID-19 at 60 and 100 days after confirmed diagnosis. We conducted a detailed questionnaire, clinical examination, laboratory testing, lung function analysis, echocardiography and thoracic low-dose computed tomography (CT).
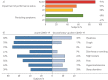
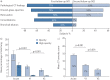
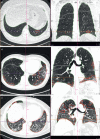
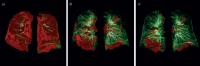
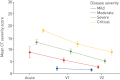
Results: Data from 145 COVID-19 patients were evaluated, and 41% of all subjects exhibited persistent symptoms 100 days after COVID-19 onset, with dyspnoea being most frequent (36%). Accordingly, patients still displayed an impaired lung function, with a reduced diffusing capacity in 21% of the cohort being the most prominent finding. Cardiac impairment, including a reduced left ventricular function or signs of pulmonary hypertension, was only present in a minority of subjects. CT scans unveiled persisting lung pathologies in 63% of patients, mainly consisting of bilateral ground-glass opacities and/or reticulation in the lower lung lobes, without radiological signs of pulmonary fibrosis. Sequential follow-up evaluations at 60 and 100 days after COVID-19 onset demonstrated a vast improvement of symptoms and CT abnormalities over time.
Conclusion: A relevant percentage of post-COVID-19 patients presented with persisting symptoms and lung function impairment along with radiological pulmonary abnormalities >100 days after the diagnosis of COVID-19. However, our results indicate a significant improvement in symptoms and cardiopulmonary status over time.
Trial registration: ClinicalTrials.gov NCT04416100.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: T. Sonnweber has nothing to disclose. Conflict of interest: S. Sahanic has nothing to disclose. Conflict of interest: A. Pizzini has nothing to disclose. Conflict of interest: A. Luger has nothing to disclose. Conflict of interest: C. Schwabl has nothing to disclose. Conflict of interest: B. Sonnweber has nothing to disclose. Conflict of interest: K. Kurz has nothing to disclose. Conflict of interest: S. Koppelstätter has nothing to disclose. Conflict of interest: D. Haschka has nothing to disclose. Conflict of interest: V. Petzer has nothing to disclose. Conflict of interest: A. Boehm has nothing to disclose. Conflict of interest: M. Aichner has nothing to disclose. Conflict of interest: P. Tymoszuk has nothing to disclose. Conflict of interest: D. Lener has nothing to disclose. Conflict of interest: M. Theurl has nothing to disclose. Conflict of interest: A. Lorsbach-Köhler has nothing to disclose. Conflict of interest: A. Tancevski has nothing to disclose. Conflict of interest: A. Schapfl has nothing to disclose. Conflict of interest: M. Schaber has nothing to disclose. Conflict of interest: R. Hilbe has nothing to disclose. Conflict of interest: M. Nairz has nothing to disclose. Conflict of interest: B. Puchner has nothing to disclose. Conflict of interest: D. Hüttenberger has nothing to disclose. Conflict of interest: C. Tschurtschenthaler has nothing to disclose. Conflict of interest: M. Aßhoff has nothing to disclose. Conflict of interest: A. Peer has nothing to disclose. Conflict of interest: F. Hartig has nothing to disclose. Conflict of interest: R. Bellmann has nothing to disclose. Conflict of interest: M. Joannidis has nothing to disclose. Conflict of interest: C. Gollmann-Tepeköylü has nothing to disclose. Conflict of interest: J. Holfeld has nothing to disclose. Conflict of interest: G. Feuchtner has nothing to disclose. Conflict of interest: A. Egger has nothing to disclose. Conflict of interest: G. Hoermann has nothing to disclose. Conflict of interest: A. Schroll has nothing to disclose. Conflict of interest: G. Fritsche has nothing to disclose. Conflict of interest: S. Wildner has nothing to disclose. Conflict of interest: R. Bellmann-Weiler has nothing to disclose. Conflict of interest: R. Kirchmair has nothing to disclose. Conflict of interest: R. Helbok has nothing to disclose. Conflict of interest: H. Prosch has nothing to disclose. Conflict of interest: D. Rieder has nothing to disclose. Conflict of interest: Z. Trajanoski has nothing to disclose. Conflict of interest: F. Kronenberg has nothing to disclose. Conflict of interest: E. Wöll has nothing to disclose. Conflict of interest: G. Weiss has nothing to disclose. Conflict of interest: G. Widmann has nothing to disclose. Conflict of interest: J. Löffler-Ragg has nothing to disclose. Conflict of interest: I. Tancevski reports an Investigator Initiated Study (IIS) grant from Boehringer Ingelheim (IIS 1199-0424).
Figures
Comment in
-
Nefer, Sinuhe and clinical research assessing post COVID-19 condition.Eur Respir J. 2021 Apr 29;57(4):2004423. doi: 10.1183/13993003.04423-2020. Print 2021 Apr. Eur Respir J. 2021. PMID: 33380509 Free PMC article.
-
Persistierende Schäden und Symptome nach COVID-19.MMW Fortschr Med. 2021 Jul;163(13):36. doi: 10.1007/s15006-021-0148-5. MMW Fortschr Med. 2021. PMID: 34240365 Free PMC article. German. No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
