Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19
- PMID: 33303540
- PMCID: PMC7736755
- DOI: 10.1183/13993003.03448-2020
Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19
Abstract
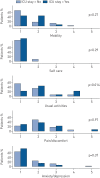
The long-term pulmonary outcomes of coronavirus disease 2019 (COVID-19) are unknown. We aimed to describe self-reported dyspnoea, quality of life, pulmonary function and chest computed tomography (CT) findings 3 months following hospital admission for COVID-19. We hypothesised outcomes to be inferior for patients admitted to intensive care units (ICUs), compared with non-ICU patients.Discharged COVID-19 patients from six Norwegian hospitals were enrolled consecutively in a prospective cohort study. The current report describes the first 103 participants, including 15 ICU patients. The modified Medical Research Council (mMRC) dyspnoea scale, the EuroQol Group's questionnaire, spirometry, diffusing capacity of the lung for carbon monoxide (D LCO), 6-min walk test, pulse oximetry and low-dose CT scan were performed 3 months after discharge.mMRC score was >0 in 54% and >1 in 19% of the participants. The median (25th-75th percentile) forced vital capacity and forced expiratory volume in 1 s were 94% (76-121%) and 92% (84-106%) of predicted, respectively. D LCO was below the lower limit of normal in 24% of participants. Ground-glass opacities (GGO) with >10% distribution in at least one of four pulmonary zones were present in 25% of participants, while 19% had parenchymal bands on chest CT. ICU survivors had similar dyspnoea scores and pulmonary function as non-ICU patients, but higher prevalence of GGO (adjusted OR 4.2, 95% CI 1.1-15.6) and lower performance in usual activities.3 months after admission for COVID-19, one-fourth of the participants had chest CT opacities and reduced diffusing capacity. Admission to ICU was associated with pathological CT findings. This was not reflected in increased dyspnoea or impaired lung function.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: T.V. Lerum has nothing to disclose. Conflict of interest: T.M. Aaløkken has nothing to disclose. Conflict of interest: E. Brønstad has nothing to disclose. Conflict of interest: B. Aarli reports personal fees for lectures and advisory board work from AstraZeneca, personal fees for lectures from GlaxoSmithKline, Novartis, Boehringer Ingelheim and Chiesi Pharma, outside the submitted work. Conflict of interest: E. Ikdahl has nothing to disclose. Conflict of interest: K.M.A. Lund has nothing to disclose. Conflict of interest: M.T. Durheim reports grants and personal fees from Boehringer Ingelheim, personal fees from Roche and AstraZeneca, outside the submitted work. Conflict of interest: J.R. Rodriguez has nothing to disclose. Conflict of interest: C. Meltzer has nothing to disclose. Conflict of interest: K. Tonby has nothing to disclose. Conflict of interest: K. Stavem has nothing to disclose. Conflict of interest: O.H. Skjønsberg has nothing to disclose. Conflict of interest: H. Ashraf reports grants from Boehringer Ingelheim, during the conduct of the study. Conflict of interest: G. Einvik reports grants from Boehringer Ingelheim, during the conduct of the study; personal fees for consultancy from AstraZeneca AB, outside the submitted work.
Figures
Comment in
-
Nefer, Sinuhe and clinical research assessing post COVID-19 condition.Eur Respir J. 2021 Apr 29;57(4):2004423. doi: 10.1183/13993003.04423-2020. Print 2021 Apr. Eur Respir J. 2021. PMID: 33380509 Free PMC article.
-
Life post-COVID-19: symptoms and chronic complications.Sao Paulo Med J. 2021 Jan-Feb;139(1):1-2. doi: 10.1590/1516-3180.2021.139104022021. Sao Paulo Med J. 2021. PMID: 33656121 Free PMC article. No abstract available.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical