Markers of disease activity in COPD: an 8-year mortality study in the ECLIPSE cohort
- PMID: 33303557
- PMCID: PMC7991608
- DOI: 10.1183/13993003.01339-2020
Markers of disease activity in COPD: an 8-year mortality study in the ECLIPSE cohort
Abstract
Rationale: There are no validated measures of disease activity in COPD. Since "active" disease is expected to have worse outcomes (e.g. mortality), we explored potential markers of disease activity in patients enrolled in the ECLIPSE cohort in relation to 8-year all-cause mortality.
Methods: We investigated 1) how changes in relevant clinical variables over time (1 or 3 years) relate to 8-year mortality; 2) whether these variables inter-relate; and 3) if any clinical, imaging and/or biological marker measured cross-sectionally at baseline relates to any activity component.
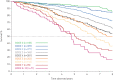
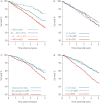
Results: Results showed that 1) after 1 year, hospitalisation for COPD, exacerbation frequency, worsening of body mass index, airflow obstruction, dyspnoea and exercise (BODE) index or health status (St George's Respiratory Questionnaire (SGRQ)) and persistence of systemic inflammation were significantly associated with 8-year mortality; 2) at 3 years, the same markers, plus forced expiratory volume in 1 s (FEV1) decline and to a lesser degree computed tomography (CT) emphysema, showed association, thus qualifying as markers of disease activity; 3) changes in FEV1, inflammatory cytokines and CT emphysema were not inter-related, while the multidimensional indices (BODE and SGRQ) showed modest correlations; and 4) changes in these markers could not be predicted by any baseline cross-sectional measure.
Conclusions: In COPD, 1- and 3-year changes in exacerbation frequency, systemic inflammation, BODE and SGRQ scores and FEV1 decline are independent markers of disease activity associated with 8-year all-cause mortality. These disease activity markers are generally independent and not predictable from baseline measurements.
Trial registration: ClinicalTrials.gov NCT00292552.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: B. Celli reports grants and personal fees for scientific committee work from GlaxoSmithKline, during the conduct of the study; grants and provision of research facilities from AstraZeneca, personal fees for consultancy and scientific committee work from GlaxoSmithKline, personal fees for consultancy from Boehringer Ingelheim, Sanofi-Aventis, Menarini, Chiesi and Pulmonx, outside the submitted work. Conflict of interest: N. Locantore is an employee and shareholder of GSK. Conflict of interest: J.C. Yates is an employee of and owns shares in GSK. Conflict of interest: P. Bakke reports personal fees for advisory board work and lectures from GlaxoSmithKline, AstraZeneca and Boehringer Ingelheim, personal fees for advisory board work from Chiesi, outside the submitted work. Conflict of interest: P.M.A. Calverley reports personal fees from GSK, Boehringer Ingelheim, Novartis, Zambon, Respironics and Recipharm, outside the submitted work. Conflict of interest: C. Crim is an employee and shareholder of GlaxoSmithKline. Conflict of interest: H.O. Coxson reports grants and personal fees for scientific committee work from GSK, during the conduct of the study. Conflict of interest: D.A. Lomas reports grants and personal fees from GlaxoSmithKline, during the conduct of the study; grants and personal fees for advisory board work and lectures from GlaxoSmithKline, personal fees for advisory board work from Griffols, outside the submitted work. Conflict of interest: W. MacNee reports personal fees for scientific committee work from GSK, during the conduct of the study; personal fees from GSK and AstraZeneca, grants and personal fees from Pfizer, outside the submitted work. Conflict of interest: B.E. Miller is an employee and shareholder of GSK. Conflict of interest: H. Mullerova is a former employee of GlaxoSmithKline. Conflict of interest: S.I. Rennard is a former employee of AstraZeneca, has provided consultancy to GSK, Verona, Bergenbio and NovoVentures, and currently holds shares in AstraZeneca, outside of the submitted work. Prior to 2007, S.I. Rennard received funding from the tobacco industry for studies relating to harm reduction and to the impact of tobacco smoke on stem cells, and also consulted with RJ Reynolds (without personal fee) on the topic of harm reduction: funding from RJ Reynolds to evaluate the effect of a harm reduction product in normal smokers (1996) and in subjects with chronic bronchitis (1999) and to assess the effect of smoking cessation on lower respiratory tract inflammation (2000); participation in a Philip Morris multicentre study to assess biomarkers of smoke exposure (2002); funding for a clinical trial from the Institute for Science and Health (2005), which receives support from the tobacco industry, to evaluate biomarkers in exhaled breath associated with smoking cessation and reduction (this study was supplemented with funding from Lorillard and RJ Reynolds); grants from the Philip Morris External Research Program (2005) to assess the impact of cigarette smoking on circulating stem cells in the mouse; consultancy for RJ Reynolds on the topic of harm reduction until 2007 (no personal remuneration). There are no active tobacco-industry funded projects. All ties with tobacco industry companies and entities supported by tobacco companies were terminated in 2007. Conflict of interest: E.K. Silverman reports grants, personal fees and travel expenses from GlaxoSmithKline, grants from NIH, during the conduct of the study. Conflict of interest: E. Wouters reports personal fees for advisory board work from Nycomed and Boehringer Ingelheim BV, grants and personal fees for lectures from AstraZeneca and GSK, personal fees for lectures from Novartis and Chiesi, outside the submitted work. Conflict of interest: R. Tal-Singer reports is an employee and shareholder of GlaxoSmithKline. Conflict of interest: A. Agusti reports personal fees for scientific committee work from GSK, during the conduct of the study; personal fees from AstraZeneca, Chiesi and Nuvaira, grants and personal fees from Menarini and GSK, outside the submitted work. Conflict of interest: J. Vestbo reports personal fees for steering committee work from GSK, during the conduct of the study; personal fees for lectures and consultancy from AstraZeneca, Chiesi and Novartis, grants and personal fees for lectures and consultancy from Boehringer Ingelheim, grants and personal fees for consultancy from GSK, outside the submitted work; and has a family member employed by Chiesi (Denmark).
Figures


Comment in
-
COPD: still an unpredictable journey.Eur Respir J. 2021 Mar 25;57(3):2002933. doi: 10.1183/13993003.02933-2020. Print 2021 Mar. Eur Respir J. 2021. PMID: 33767001 No abstract available.
References
-
- GBD Cause of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736–1788. doi:10.1016/S0140-6736(18)32203-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
