Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength
- PMID: 33303683
- PMCID: PMC7938328
- DOI: 10.1126/science.abc8059
Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength
Abstract
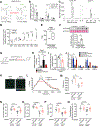
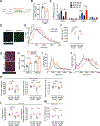
Treatments are lacking for sarcopenia, a debilitating age-related skeletal muscle wasting syndrome. We identifed increased amounts of 15-hydroxyprostaglandin dehydrogenase (15-PGDH), the prostaglandin E2 (PGE2)-degrading enzyme, as a hallmark of aged tissues, including skeletal muscle. The consequent reduction in PGE2 signaling contributed to muscle atrophy in aged mice and results from 15-PGDH-expressing myofibers and interstitial cells, such as macrophages, within muscle. Overexpression of 15-PGDH in young muscles induced atrophy. Inhibition of 15-PGDH, by targeted genetic depletion or a small-molecule inhibitor, increased aged muscle mass, strength, and exercise performance. These benefits arise from a physiological increase in PGE2 concentrations, which augmented mitochondrial function and autophagy and decreased transforming growth factor-β signaling and activity of ubiquitin-proteasome pathways. Thus, PGE2 signaling ameliorates muscle atrophy and rejuvenates muscle function, and 15-PGDH may be a suitable therapeutic target for countering sarcopenia.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures






Comment in
-
Targeting enzyme aging.Science. 2021 Jan 29;371(6528):462-463. doi: 10.1126/science.abf9566. Science. 2021. PMID: 33510013 No abstract available.
References
-
- Cohen S, Nathan JA, Goldberg AL, Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 14, 58–74 (2015). - PubMed
-
- Lee CE, McArdle A, Griffiths RD, The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr 26, 524–534 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

