The vascular endothelial growth factor trap aflibercept induces vascular dysfunction and hypertension via attenuation of eNOS/NO signaling in mice
- PMID: 33303990
- PMCID: PMC8379246
- DOI: 10.1038/s41401-020-00569-1
The vascular endothelial growth factor trap aflibercept induces vascular dysfunction and hypertension via attenuation of eNOS/NO signaling in mice
Abstract
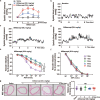
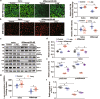
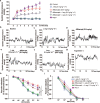
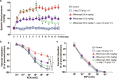
Aflibercept, as a soluble decoy vascular endothelial growth factor receptor, Which has been used as a first-line monotherapy for cancers. Aflibercept often causes cardiovascular toxicities including hypertension, but the mechanisms underlying aflibercept-induced hypertension remain unknown. In this study we investigated the effect of short-term and long-term administration of aflibercept on blood pressure (BP), vascular function, NO bioavailability, oxidative stress and endothelin 1 (ET-1) in mice and cultured endothelial cells. We showed that injection of a single-dose of aflibercept (18.2, 36.4 mg/kg, iv) rapidly and dose-dependently elevated BP in mice. Aflibercept treatment markedly impaired endothelial-dependent relaxation (EDR) and resulted in NADPH oxidases 1 (NOX1)- and NADPH oxidases 4 (NOX4)-mediated generation of ROS, decreased the activation of protein kinase B (Akt) and endothelial nitric oxide synthase (eNOS) concurrently with a reduction in nitric oxide (NO) production and elevation of ET-1 levels in mouse aortas; these effects were greatly attenuated by supplementation of L-arginine (L-arg, 0.5 or 1.0 g/kg, bid, ig) before aflibercept injection. Similar results were observed in L-arg-pretreated cultured endothelial cells, showing markedly decreased ROS accumulation and AKT/eNOS/NO signaling impairment induced by aflibercept. In order to assess the effects of long-term aflibercept on hypertension and to evaluate the beneficial effects of L-arg supplementation, we administered these two drugs to WT mice for up to 14 days (at an interval of two days). Long-term administration of aflibercept resulted in a sustained increase in BP and a severely impaired EDR, which are associated with NOX1/NOX4-mediated production of ROS, increase in ET-1, inhibition of AKT/eNOS/NO signaling and a decreased expression of cationic amino acid transporter (CAT-1). The effects caused by long-term administration were greatly attenuated by L-arg supplementation in a dose-dependent manner. We conclude that aflibercept leads to vascular dysfunction and hypertension by inhibiting CAT-1/AKT/eNOS/NO signaling, increasing ET-1, and activating NOX1/NOX4-mediated oxidative stress, which can be suppressed by supplementation of L-arg. Therefore, L-arg could be a potential therapeutic agent for aflibercept-induced hypertension.
Keywords: L-arginine; cultured endothelial cells; aflibercept; endothelial dysfunction; hypertension; superoxide production; endothelin 1; cationic amino acid transporter-1.
© 2020. CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

