Exposure to Artificial Light at Night and the Consequences for Flora, Fauna, and Ecosystems
- PMID: 33304237
- PMCID: PMC7701298
- DOI: 10.3389/fnins.2020.602796
Exposure to Artificial Light at Night and the Consequences for Flora, Fauna, and Ecosystems
Abstract
The present review draws together wide-ranging studies performed over the last decades that catalogue the effects of artificial-light-at-night (ALAN) upon living species and their environment. We provide an overview of the tremendous variety of light-detection strategies which have evolved in living organisms - unicellular, plants and animals, covering chloroplasts (plants), and the plethora of ocular and extra-ocular organs (animals). We describe the visual pigments which permit photo-detection, paying attention to their spectral characteristics, which extend from the ultraviolet into infrared. We discuss how organisms use light information in a way crucial for their development, growth and survival: phototropism, phototaxis, photoperiodism, and synchronization of circadian clocks. These aspects are treated in depth, as their perturbation underlies much of the disruptive effects of ALAN. The review goes into detail on circadian networks in living organisms, since these fundamental features are of critical importance in regulating the interface between environment and body. Especially, hormonal synthesis and secretion are often under circadian and circannual control, hence perturbation of the clock will lead to hormonal imbalance. The review addresses how the ubiquitous introduction of light-emitting diode technology may exacerbate, or in some cases reduce, the generalized ever-increasing light pollution. Numerous examples are given of how widespread exposure to ALAN is perturbing many aspects of plant and animal behaviour and survival: foraging, orientation, migration, seasonal reproduction, colonization and more. We examine the potential problems at the level of individual species and populations and extend the debate to the consequences for ecosystems. We stress, through a few examples, the synergistic harmful effects resulting from the impacts of ALAN combined with other anthropogenic pressures, which often impact the neuroendocrine loops in vertebrates. The article concludes by debating how these anthropogenic changes could be mitigated by more reasonable use of available technology - for example by restricting illumination to more essential areas and hours, directing lighting to avoid wasteful radiation and selecting spectral emissions, to reduce impact on circadian clocks. We end by discussing how society should take into account the potentially major consequences that ALAN has on the natural world and the repercussions for ongoing human health and welfare.
Keywords: anthropogenic impact; artificial-light-at-night; biological clocks; ecosystems; light-emitting-diodes; photoreception.
Copyright © 2020 Falcón, Torriglia, Attia, Viénot, Gronfier, Behar-Cohen, Martinsons and Hicks.
Figures


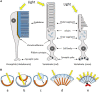


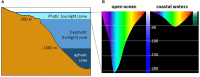
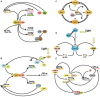
 are present in the pineal and retinal photoreceptors as well as in the basal diencephalon (preoptic area [POA] and suprachiasmatic nuclei [SCN]) of lizards and birds. The pineal gland of fish and lizards also integrates temperature information from the external environment. The concomitant action of light, temperature and other internal factors, shapes the rhythmic nervous (blue) and hormonal (red; melatonin) outputs (see text for details), providing a temporal message transmitted to the neuroendocrine axis and downstream targets (peripheral endocrine organs). Melatonin acts through specific receptors (stars) distributed in different tissues and organs. While the main retinal output subserves visual function, a few other fibres also terminate in different parts of the basal diencephalon, where some converge with fibres originating from the pineal gland. Some of the targeted areas also express melatonin receptors. This double or triple input contributes to synchronizing the neuronal activity of the basal diencephalon. In sauropsids the POA and SCN neurons also relay retinal information to the pineal gland. The entire neuroendocrine axis is targeted by ALAN together with multiple other disruptors including temperature rises and pollutants [e.g., endocrine disruptors] acting directly or indirectly at different levels of the loop.
are present in the pineal and retinal photoreceptors as well as in the basal diencephalon (preoptic area [POA] and suprachiasmatic nuclei [SCN]) of lizards and birds. The pineal gland of fish and lizards also integrates temperature information from the external environment. The concomitant action of light, temperature and other internal factors, shapes the rhythmic nervous (blue) and hormonal (red; melatonin) outputs (see text for details), providing a temporal message transmitted to the neuroendocrine axis and downstream targets (peripheral endocrine organs). Melatonin acts through specific receptors (stars) distributed in different tissues and organs. While the main retinal output subserves visual function, a few other fibres also terminate in different parts of the basal diencephalon, where some converge with fibres originating from the pineal gland. Some of the targeted areas also express melatonin receptors. This double or triple input contributes to synchronizing the neuronal activity of the basal diencephalon. In sauropsids the POA and SCN neurons also relay retinal information to the pineal gland. The entire neuroendocrine axis is targeted by ALAN together with multiple other disruptors including temperature rises and pollutants [e.g., endocrine disruptors] acting directly or indirectly at different levels of the loop.


References
-
- Acharya J., Rechner O., Neugart S., Schreiner M., Poehling H. M. (2016). Effects of light-emitting diode treatments on Brevicoryne brassicae performance mediated by secondary metabolites in Brussels sprouts. J. Plant Dis. Protec. 123 321–330. 10.1007/s41348-016-0029-9 - DOI
-
- Alsanius B. W., Karlsson M., Rosberg A. K., Dorais M., Naznin M. T., Khalil S., et al. (2019). Light and microbial lifestyle: the impact of light quality on plant-microbe interactions in horticultural production systems-a review. Horticulturae 5:41 10.3390/horticulturae5020041 - DOI
-
- Altringham J., Kerth G. (2015). “Bats and Roads,” in Bats in the Anthropocene: Conservation of Bats in a Changing World, eds Voigt C., Kingston T. (Cham: Springer; ), 35–62.
Publication types
LinkOut - more resources
Full Text Sources

