Uric Acid and Cardiovascular Disease: An Update From Molecular Mechanism to Clinical Perspective
- PMID: 33304270
- PMCID: PMC7701250
- DOI: 10.3389/fphar.2020.582680
Uric Acid and Cardiovascular Disease: An Update From Molecular Mechanism to Clinical Perspective
Abstract
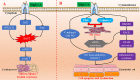
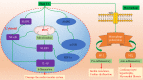
Uric acid (UA) is the end product of purine nucleotide metabolism in the human body. Hyperuricemia is an abnormally high level of UA in the blood and may result in arthritis and gout. The prevalence of hyperuricemia has been increasing globally. Epidemiological studies have shown that UA levels are positively correlated with cardiovascular diseases, including hypertension, atherosclerosis, atrial fibrillation (AF), and heart failure (HF). Hyperuricemia promotes the occurrence and development of cardiovascular diseases by regulating molecular signals, such as inflammatory response, oxidative stress, insulin resistance/diabetes, endoplasmic reticulum stress, and endothelial dysfunction. Despite extensive research, the underlying molecular mechanisms are still unclear. Allopurinol, a xanthine oxidase (XO) inhibitor, has been shown to improve cardiovascular outcomes in patients with HF, coronary heart disease (CHD), type 2 diabetes (T2D), and left ventricular hypertrophy (LVH). Whether febuxostat, another XO inhibitor, can improve cardiovascular outcomes as well as allopurinol remains controversial. Furthermore, it is also not clear whether UA-lowering treatment (ULT) can benefit patients with asymptomatic hyperuricemia. In this review, we focus on the latest cellular and molecular findings of cardiovascular disease associated with hyperuricemia and clinical data about the efficacy of ULT in patients with cardiovascular disease.
Keywords: cardiovascular disease; clinical prospect; molecular mechanism; therapeutics; uric acid.
Copyright © 2020 Yu and Cheng.
Figures

References
-
- Bailey C. J., Gross J. L., Pieters A., Bastien A., List J. F. (2010). Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375 (9733), 2223–2233. 10.1016/S0140-6736(10)60407-2 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

