mTOR Pathway in Gastroenteropancreatic Neuroendocrine Tumor (GEP-NETs)
- PMID: 33304317
- PMCID: PMC7701056
- DOI: 10.3389/fendo.2020.562505
mTOR Pathway in Gastroenteropancreatic Neuroendocrine Tumor (GEP-NETs)
Abstract
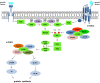
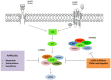
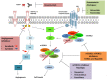
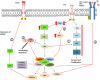
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) originate from neuroendocrine cells in the gastrointestinal tract. They are heterogeneous, and though initially considered rare tumors, the incidence of GEP-NENs has increased in the last few decades. Therapeutic approaches for the metastatic disease include surgery, radiological intervention by chemoembolisation, radiofrequency ablation, biological therapy in addition to somatostatin analogs, and PRRT therapy (177Lu-DOTATATE). The PI3K-AKT-mTOR pathway is essential in the regulation of protein translation, cell growth, and metabolism. Evidence suggests that the mTOR pathway is involved in malignant progression and resistance to treatment through over-activation of several mechanisms. PI3K, one of the main downstream of the Akt-mTOR axis, is mainly involved in the neoplastic process. This pathway is frequently deregulated in human tumors, making it a central target in the development of new anti-cancer treatments. Recent molecular studies identify potential targets within the PI3K/Akt/mTOR pathway in GEP-NENs. However, the use of target therapy has been known to lead to resistance due to several mechanisms such as feedback activation of alternative pathways, inactivation of protein kinases, and deregulation of the downstream mTOR components. Therefore, the specific role of targeted drugs for the management of GEP-NENs is yet to be well-defined. The variable clinical presentation of advanced neuroendocrine tumors is a significant challenge for designing studies. This review aims to highlight the role of the PI3K/Akt/mTOR pathway in the development of neuroendocrine tumors and further specify its potential as a therapeutic target in advanced stages.
Keywords: GEP-NENs; GEP-NETs; cancer treatment; mTOR; neuroendocrine tumor; target therapy.
Copyright © 2020 Zanini, Renzi, Giovinazzo and Bermano.
Figures
Similar articles
-
Anti-proliferative and anti-secretory effects of everolimus on human pancreatic neuroendocrine tumors primary cultures: is there any benefit from combination with somatostatin analogs?Oncotarget. 2017 Jun 20;8(25):41044-41063. doi: 10.18632/oncotarget.17008. Oncotarget. 2017. PMID: 28454119 Free PMC article.
-
Resistance to targeted treatment of gastroenteropancreatic neuroendocrine tumors.Endocr Relat Cancer. 2019 Mar 1;26(3):R109-R130. doi: 10.1530/ERC-18-0420. Endocr Relat Cancer. 2019. PMID: 32022503 Review.
-
Prognostic and predictive role of the PI3K-AKT-mTOR pathway in neuroendocrine neoplasms.Clin Transl Oncol. 2018 May;20(5):561-569. doi: 10.1007/s12094-017-1758-3. Epub 2017 Nov 9. Clin Transl Oncol. 2018. PMID: 29124519 Review.
-
Advances in the systemic treatment of neuroendocrine tumors in the era of molecular therapy.Anticancer Agents Med Chem. 2013 Mar;13(3):382-8. Anticancer Agents Med Chem. 2013. PMID: 23092266 Review.
-
Antitumor effect of everolimus in preclinical models of high-grade gastroenteropancreatic neuroendocrine carcinomas.Neuroendocrinology. 2013;97(4):331-40. doi: 10.1159/000347063. Epub 2013 May 22. Neuroendocrinology. 2013. PMID: 23343749
Cited by
-
Surgical Management and Long-Term Evaluation of Pancreatic Neuroendocrine Tumors.Surg Clin North Am. 2024 Aug;104(4):891-908. doi: 10.1016/j.suc.2024.02.019. Epub 2024 Apr 4. Surg Clin North Am. 2024. PMID: 38944507 Free PMC article. Review.
-
Combined deletion of MEN1, ATRX and PTEN triggers development of high-grade pancreatic neuroendocrine tumors in mice.Sci Rep. 2024 Apr 12;14(1):8510. doi: 10.1038/s41598-024-58874-2. Sci Rep. 2024. PMID: 38609433 Free PMC article.
-
Management of Appendix Neuroendocrine Neoplasms: Insights on the Current Guidelines.Cancers (Basel). 2022 Dec 31;15(1):295. doi: 10.3390/cancers15010295. Cancers (Basel). 2022. PMID: 36612291 Free PMC article. Review.
-
Predictive Factors for Resistant Disease with Medical/Radiologic/Liver-Directed Anti-Tumor Treatments in Patients with Advanced Pancreatic Neuroendocrine Neoplasms: Recent Advances and Controversies.Cancers (Basel). 2022 Feb 28;14(5):1250. doi: 10.3390/cancers14051250. Cancers (Basel). 2022. PMID: 35267558 Free PMC article. Review.
-
Adverse Events of Radioligand Therapy in Patients with Progressive Neuroendocrine Neoplasms: The Biggest Eastern European Prospective Study.Cancers (Basel). 2024 Oct 17;16(20):3509. doi: 10.3390/cancers16203509. Cancers (Basel). 2024. PMID: 39456603 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

