CCN3 Signaling Is Differently Regulated in Placental Diseases Preeclampsia and Abnormally Invasive Placenta
- PMID: 33304321
- PMCID: PMC7701218
- DOI: 10.3389/fendo.2020.597549
CCN3 Signaling Is Differently Regulated in Placental Diseases Preeclampsia and Abnormally Invasive Placenta
Abstract
Objectives: An adequate development of the placenta includes trophoblast differentiation with the processes of trophoblast migration, invasion, cellular senescence and apoptosis which are all crucial to establishing a successful pregnancy. Altered placental development and function lead to placental diseases such as preeclampsia (PE) which is mainly characterized by insufficient trophoblast invasion and abnormally invasive placenta (AIP) disorders (Placenta accreta, increta, or percreta) which are characterized by excessive trophoblast invasion. Both of them will cause maternal and fetal morbidity/mortality. However, the etiology of these diseases is still unclear. Our previous study has shown that the matricellular protein nephroblastoma overexpressed (NOV, CCN3) induces G0/G1 cell cycle arrest, drives trophoblast cells into senescence and activates FAK and Akt kinases resulting in reduced cell proliferation and enhanced migration capability of the human trophoblast cell line SGHPL-5. The present study focuses on whether CCN3 can alter cell cycle-regulated pathways associated with trophoblast senescence and invasion activity in pathological versus gestational age-matched control placentas.
Methods: Cell cycle regulator proteins were investigated by immunoblotting and qPCR. For localization of CCN3, p16, p21, and Cyclin D1 proteins, co-immunohistochemistry was performed.
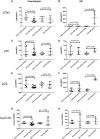
Results: In early-onset PE placentas, CCN3 was expressed at a significantly lower level compared to gestational age-matched controls. The decrease of CCN3 level is associated with an increase in p53, Cyclin E1 and pRb protein expression, whereas the level of cleaved Notch-1, p21, Cyclin D1, pFAK, pAKT, and pmTOR protein decreased. In term AIP placentas, the expression of CCN3 was significantly increased compared to matched term controls. This increase was correlated to an increase in p53, p16, p21, Cyclin D1, cleaved Notch-1, pFAK, pAkt, and pmTOR whereas pRb was significantly decreased. However, in late PE and early AIP placentas, no significant differences in CCN3, p16, p21, Cyclin D1, p53, and cleaved Notch-1 expression were found when matched to appropriate controls.
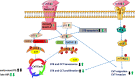
Conclusions: CCN3 expression levels are correlated to markers of cell cycle arrest oppositely in PE and AIP by activating the FAK/AKT pathway in AIP or down-regulating in PE. This may be one mechanism to explain the different pathological features of placental diseases, PE and AIP.
Keywords: CCN3; abnormally invasive placenta; invasion; preeclampsia; senescence; trophoblast.
Copyright © 2020 Duan, Schimmelmann, Wu, Reisch, Faas, Kimmig, Winterhager, Köninger and Gellhaus.
Figures







Similar articles
-
CCN1 (CYR61) and CCN3 (NOV) signaling drives human trophoblast cells into senescence and stimulates migration properties.Cell Adh Migr. 2016 Mar 3;10(1-2):163-78. doi: 10.1080/19336918.2016.1139265. Epub 2016 Jan 8. Cell Adh Migr. 2016. PMID: 26744771 Free PMC article.
-
CCN3 regulates proliferation and migration properties in Jeg3 trophoblast cells via ERK1/2, Akt and Notch signalling.Mol Hum Reprod. 2013 Apr;19(4):237-49. doi: 10.1093/molehr/gas061. Epub 2012 Dec 6. Mol Hum Reprod. 2013. PMID: 23220688
-
Downregulation of receptor tyrosine kinase-like orphan receptor 1 in preeclampsia placenta inhibits human trophoblast cell proliferation, migration, and invasion by PI3K/AKT/mTOR pathway accommodation.Placenta. 2019 Jul;82:17-24. doi: 10.1016/j.placenta.2019.05.002. Epub 2019 May 12. Placenta. 2019. PMID: 31174622
-
Research Progress on Extracellular Matrix Involved in the Development of Preeclampsia.Curr Protein Pept Sci. 2024;25(7):527-538. doi: 10.2174/0113892037284176240302052521. Curr Protein Pept Sci. 2024. PMID: 38561606 Review.
-
Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction?Am J Obstet Gynecol. 2018 Feb;218(2S):S762-S773. doi: 10.1016/j.ajog.2017.11.567. Epub 2017 Dec 22. Am J Obstet Gynecol. 2018. PMID: 29275823 Review.
Cited by
-
Cellular communication network factor 3 in cartilage development and maintenance.J Cell Commun Signal. 2021 Dec;15(4):533-543. doi: 10.1007/s12079-021-00629-z. Epub 2021 Jun 14. J Cell Commun Signal. 2021. PMID: 34125392 Free PMC article. Review.
-
Deciphering Shared Gene Signatures and Immune Infiltration Characteristics Between Gestational Diabetes Mellitus and Preeclampsia by Integrated Bioinformatics Analysis and Machine Learning.Reprod Sci. 2025 Jun;32(6):1886-1904. doi: 10.1007/s43032-025-01847-1. Epub 2025 May 15. Reprod Sci. 2025. PMID: 40374866
-
High TXNIP expression accelerates the migration and invasion of the GDM placenta trophoblast.BMC Pregnancy Childbirth. 2023 Apr 10;23(1):235. doi: 10.1186/s12884-023-05524-6. BMC Pregnancy Childbirth. 2023. PMID: 37038114 Free PMC article.
-
Decidualization of human endometrial stromal cells requires steroid receptor coactivator-3.Front Reprod Health. 2022 Nov 24;4:1033581. doi: 10.3389/frph.2022.1033581. eCollection 2022. Front Reprod Health. 2022. PMID: 36505394 Free PMC article.
-
Identification of altered miRNAs and their targets in placenta accreta.Front Endocrinol (Lausanne). 2023 Mar 3;14:1021640. doi: 10.3389/fendo.2023.1021640. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36936174 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

