Primary ciliary dyskinesia: a major player in a bigger game
- PMID: 33304404
- PMCID: PMC7714554
- DOI: 10.1183/20734735.0047-2020
Primary ciliary dyskinesia: a major player in a bigger game
Abstract
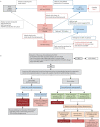
Primary ciliary dyskinesia (PCD) is an inherited disorder of clinical and genetic heterogeneity resulting from mutations in genes involved in the transport, assembly and function of motile cilia. The resulting impairment in mucociliary clearance means patients suffer from chronic progressive lung disease, bronchiectasis, rhinosinusitis and middle ear disease. Subfertility is common to both male and female patients. Situs abnormalities occur in around half of patients, with a subgroup suffering more complex situs arrangements where congenital heart defects or other organ abnormalities frequently coexist. Variations from the classical PCD phenotype are increasingly recognised where overlapping features across a range of motile and nonmotile ciliopathies are redefining our approach to both diagnosis and management of these complex conditions. PCD offers an ideal opportunity for direct visualisation of ciliary function and structure, following nasal brush biopsy, allowing opportunities for researchers to directly interrogate the downstream impact of loss of function mutations. In turn, this has led to rapid advances in the development of new diagnostic tests. These advances mean that PCD is an excellent disease model for understanding the genetic and mechanistic causes of the clinical phenotype for all respiratory ciliopathies. Furthermore, the overlapping role of motile ciliary defects in a wider set of complex and syndromic disorders related to loss of function mutations in primary, nonmotile cilia has been recognised. As we better understand the role of ciliary defects in a broad spectrum of diseases, we should aim to map out a framework through which we can identify, diagnose and treat all respiratory ciliopathies.
Key points: Primary ciliary dyskinesia is just one of a group of conditions where a heterogeneous array of genetic mutations affect the assembly or structure of motile cilia.Overlapping phenotypes between motile and nonmotile ciliopathies are redefining the diagnostic and therapeutic approach to encompass all ciliopathy patients with a respiratory phenotype.An extended diagnostic algorithm may be required to capture the majority of cases with a respiratory ciliopathy, including patients with syndromic ciliopathies.The terminology around disorders of motile cilia is becoming more descriptive to better reflect the heterogeneity and underlying disease mechanisms across the spectrum of respiratory ciliopathies.
Educational aims: To summarise the existing knowledge base around the disease mechanisms for respiratory ciliopathies, including primary ciliary dyskinesia (PCD).To explore and understand the reasons for changing terminology around respiratory ciliopathies.To emphasise key messages around the diagnosis and treatment of all ciliopathies.Diagnosing PCD is complex and time consuming, and there is no single stand-alone test that can confirm or exclude a diagnosis in all cases.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: C. Hogg has nothing to disclose. Conflict of interest: R. Bhatt has nothing to disclose.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
