EGFR-rich extracellular vesicles derived from highly metastatic nasopharyngeal carcinoma cells accelerate tumour metastasis through PI3K/AKT pathway-suppressed ROS
- PMID: 33304472
- PMCID: PMC7710133
- DOI: 10.1002/jev2.12003
EGFR-rich extracellular vesicles derived from highly metastatic nasopharyngeal carcinoma cells accelerate tumour metastasis through PI3K/AKT pathway-suppressed ROS
Abstract
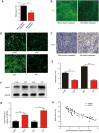
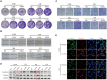
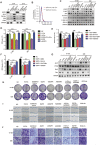
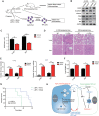
Nasopharyngeal carcinoma (NPC) is the most common cancer with high metastatic potential that occurs in the epithelial cells of the nasopharynx. Distant metastases are the primary cause for treatment failure and mortality of NPC patients. However, the underlying mechanism responsible for the initiation of tumour cell dissemination and tumour metastasis in NPC is not well understood. Here, we demonstrated that epidermal growth factor receptor (EGFR) was highly expressed in tumour tissues of NPC patients with distant metastases and was associated with a decrease in reactive oxygen species (ROS). We also revealed that extracellular vesicles (EVs) transfer occurred from highly to poorly metastatic NPC cells, mediating cell-cell communication and enhancing the metastatic potential of poorly metastatic NPC cells. Further experiments indicated that EVs derived from highly metastatic NPC cells induced the up-regulation of EGFR and down-regulation of ROS in low metastatic NPC cells. Mechanistically, EGFR-rich EVs-mediated EGFR overexpression down-regulated intracellular ROS levels through the PI3K/AKT pathway, thus promoting the metastatic potential of poorly metastatic NPC cells. Strikingly, treatment with EVs secreted from highly metastatic NPC cells was significantly associated with rapid NPC progression and shorter survival in xenografted mice. These findings not only improve our understanding of EVs-mediated NPC metastatic mechanism but also have important implications for the detection and treatment of NPC patients accompanied by aberrant EGFR-rich EVs transmission.
Keywords: epidermal growth factor receptor; extracellular vesicles; nasopharyngeal carcinoma; reactive oxygen species.
© 2020 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflict of interest.
Figures


Similar articles
-
FLT1-enriched extracellular vesicles induce a positive feedback loop between nasopharyngeal carcinoma cells and endothelial cells to promote angiogenesis and tumour metastasis.Oncogene. 2025 Jul;44(27):2253-2267. doi: 10.1038/s41388-025-03389-x. Epub 2025 Apr 13. Oncogene. 2025. PMID: 40223024
-
Four-dimensional trapped ion mobility spectrometry proteomics reveals circulating extracellular vesicles encapsulated drivers of nasopharyngeal carcinoma distant dissemination.Talanta. 2025 Jan 1;282:126907. doi: 10.1016/j.talanta.2024.126907. Epub 2024 Sep 18. Talanta. 2025. PMID: 39341061
-
Platelet-derived extracellular vesicles inhibit ferroptosis and promote distant metastasis of nasopharyngeal carcinoma by upregulating ITGB3.Int J Biol Sci. 2022 Sep 24;18(15):5858-5872. doi: 10.7150/ijbs.76162. eCollection 2022. Int J Biol Sci. 2022. PMID: 36263165 Free PMC article.
-
The role of extracellular vesicles in the development of nasopharyngeal carcinoma and potential clinical applications.Cancer Med. 2023 Jul;12(13):14484-14497. doi: 10.1002/cam4.6099. Epub 2023 Jun 12. Cancer Med. 2023. PMID: 37306659 Free PMC article. Review.
-
Extracellular vesicle-associated organotropic metastasis.Cell Prolif. 2021 Jan;54(1):e12948. doi: 10.1111/cpr.12948. Epub 2020 Nov 3. Cell Prolif. 2021. PMID: 33145869 Free PMC article. Review.
Cited by
-
Tumor metastasis: Mechanistic insights and therapeutic interventions.MedComm (2020). 2021 Dec 2;2(4):587-617. doi: 10.1002/mco2.100. eCollection 2021 Dec. MedComm (2020). 2021. PMID: 34977870 Free PMC article. Review.
-
Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through Ephrin A1-EPHA2 forward signaling.Theranostics. 2022 May 13;12(9):4127-4146. doi: 10.7150/thno.72404. eCollection 2022. Theranostics. 2022. PMID: 35673569 Free PMC article.
-
Nasopharyngeal Carcinoma: The Role of the EGFR in Epstein-Barr Virus Infection.Pathogens. 2021 Aug 31;10(9):1113. doi: 10.3390/pathogens10091113. Pathogens. 2021. PMID: 34578147 Free PMC article. Review.
-
Crosstalk between Oxidative Stress and Exosomes.Oxid Med Cell Longev. 2022 Aug 30;2022:3553617. doi: 10.1155/2022/3553617. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36082080 Free PMC article. Review.
-
KAP1 phosphorylation promotes the survival of neural stem cells after ischemia/reperfusion by maintaining the stability of PCNA.Stem Cell Res Ther. 2022 Jul 7;13(1):290. doi: 10.1186/s13287-022-02962-5. Stem Cell Res Ther. 2022. PMID: 35799276 Free PMC article.
References
-
- Cairns, R. A. , Harris, I. S , & Mak, T. W. (2011). Regulation of cancer cell metabolism. Nature Reviews Cancer, 11(2), 85–95. - PubMed
-
- Chan, K. C. , Hung, E. C. , Woo, J. K. , Chan, P. K. S. , Leung, S.‐F. , Lai, F. P. T. , … Lo, Y. M. D. (2013). Early detection of nasopharyngeal carcinoma by plasma Epstein‐Barr virus DNA analysis in a surveillance program. Cancer, 119(10), 1838–1844. - PubMed
-
- Chan, Y. K. , Zhang, H. , Liu, P. , Tsao, S.‐W. , Lung, M. L. , Mak, N.‐K. , … Yue, P. Y.‐K. (2015). Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. International Journal Of Cancer. Journal International du Cancer, 137(8), 1830–1841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

