Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25
- PMID: 33304476
- PMCID: PMC7710129
- DOI: 10.1002/jev2.12024
Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25
Abstract
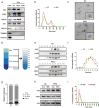
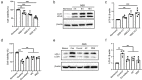
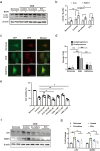
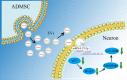
Grafted mesenchymal stem cells (MSCs) yield neuroprotection in preclinical stroke models by secreting extracellular vesicles (EVs). The neuroprotective cargo of EVs, however, has not yet been identified. To investigate such cargo and its underlying mechanism, primary neurons were exposed to oxygen-glucose-deprivation (OGD) and cocultured with adipose-derived MSCs (ADMSCs) or ADMSC-secreted EVs. Under such conditions, both ADMSCs and ADMSC-secreted EVs significantly reduced neuronal death. Screening for signalling cascades being involved in the interaction between ADMSCs and neurons revealed a decreased autophagic flux as well as a declined p53-BNIP3 activity in neurons receiving either treatment paradigm. However, the aforementioned effects were reversed when ADMSCs were pretreated with the inhibitor of exosomal secretion GW4869 or when Hrs was knocked down. In light of miR-25-3p being the most highly expressed miRNA in ADMSC-EVs interacting with the p53 pathway, further in vitro work focused on this pathway. Indeed, a miR-25-3p oligonucleotide mimic reduced cell death, whereas the anti-oligonucleotide increased autophagic flux and cell death by modulating p53-BNIP3 signalling in primary neurons exposed to OGD. Likewise, native ADMSC-EVs but not EVs obtained from ADMSCs pretreated with the anti-miR-25-3p oligonucleotide (ADMSC-EVsanti-miR-25-3p) confirmed the aforementioned in vitro observations in C57BL/6 mice exposed to cerebral ischemia. The infarct size was reduced, and neurological recovery was increased in mice treated with native ADMSC-EVs when compared to ADMSC-EVsanti-miR-25-3p. ADMSCs induce neuroprotection by improved autophagic flux through secreted EVs containing miR-25-3p. Hence, our work uncovers a novel key factor in naturally secreted ADMSC-EVs for the regulation of autophagy and induction of neuroprotection in a preclinical stroke model.
Keywords: autophagy; cerebral ischemia; extracellular vesicles, adipose‐derived MSCs; neurological recovery.
© 2020 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare to have no conflict of interest of any kind.
Figures




References
-
- Ahmadian Kia, N. , Bahrami, A. R. , Ebrahimi, M. , Matin, M. M. , Neshati, Z. , Almohaddesin, M. R. , … Bidkhori, H. R. (2011). Comparative analysis of chemokine receptor's expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. Journal of Molecular Neuroscience, 44(3), 178–185. - PubMed
-
- Althaus, J. , Bernaudin, M. , Petit, E. , Toutain, J. , Touzani, O. , & Rami, A. (2006). Expression of the gene encoding the pro‐apoptotic BNIP3 protein and stimulation of hypoxia‐inducible factor‐1alpha (HIF‐1alpha) protein following focal cerebral ischemia in rats. Neurochemistry International, 48(8), 687–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

