Human-engineered Treg-like cells suppress FOXP3-deficient T cells but preserve adaptive immune responses in vivo
- PMID: 33304583
- PMCID: PMC7688376
- DOI: 10.1002/cti2.1214
Human-engineered Treg-like cells suppress FOXP3-deficient T cells but preserve adaptive immune responses in vivo
Abstract
Objectives: Genetic or acquired defects in FOXP3+ regulatory T cells (Tregs) play a key role in many immune-mediated diseases including immune dysregulation polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Previously, we demonstrated CD4+ T cells from healthy donors and IPEX patients can be converted into functional Treg-like cells by lentiviral transfer of FOXP3 (CD4LVFOXP3). These CD4LVFOXP3 cells have potent regulatory function, suggesting their potential as an innovative therapeutic. Here, we present molecular and preclinical in vivo data supporting CD4LVFOXP3 cell clinical progression.
Methods: The molecular characterisation of CD4LVFOXP3 cells included flow cytometry, qPCR, RNA-seq and TCR-seq. The in vivo suppressive function of CD4LVFOXP3 cells was assessed in xenograft-versus-host disease (xeno-GvHD) and FOXP3-deficient IPEX-like humanised mouse models. The safety of CD4LVFOXP3 cells was evaluated using peripheral blood (PB) humanised (hu)- mice testing their impact on immune response against pathogens, and immune surveillance against tumor antigens.
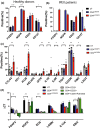
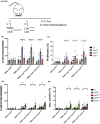
Results: We demonstrate that the conversion of CD4+ T cells to CD4LVFOXP3 cells leads to specific transcriptional changes as compared to CD4+ T-cell transduction in the absence of FOXP3, including upregulation of Treg-related genes. Furthermore, we observe specific preservation of a polyclonal TCR repertoire during in vitro cell production. Both allogeneic and autologous CD4LVFOXP3 cells protect from xeno-GvHD after two sequential infusions of effector T cells. CD4LVFOXP3 cells prevent hyper-proliferation of CD4+ memory T cells in the FOXP3-deficient IPEX-like hu-mice. CD4LVFOXP3 cells do not impede in vivo expansion of antigen-primed T cells or tumor clearance in the PB hu-mice.
Conclusion: These data support the clinical readiness of CD4LVFOXP3 cells to treat IPEX syndrome and other immune-mediated diseases caused by insufficient or dysfunctional FOXP3+ Tregs.
Keywords: CRISPR/Cas9; FOXP3; IPEX syndrome; gene therapy; lentiviral vector; regulatory T cells.
© 2020 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest. REFERENCES1SakaguchiS, SakaguchiN, AsanoM, ItohM, TodaM. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol1995; 155: 1151–1164.76361842SakaguchiS, MiyaraM, CostantinoCM, HaflerDA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol2010; 10: 490–500.205593273HoriS, NomuraT, SakaguchiS. Control of regulatory T cell development by the transcription factor Foxp3. Science2003; 299: 1057–1061.125222564FontenotJD, GavinMA, RudenskyAY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol2003; 4: 330–336.126125785DugglebyR, DanbyRD, MadrigalJA, SaudemontA. Clinical grade regulatory CD4+ T cells (Tregs): moving toward cellular‐based immunomodulatory therapies. Front Immunol2018; 9: 252.294876026BluestoneJA, BucknerJH, FitchMet alType 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med2015; 7: 315ra189.7FerreiraLMR, MullerYD, BluestoneJA, TangQ. Next‐generation regulatory T cell therapy. Nat Rev Drug Discov2019; 18: 749–769.315412248BacchettaR, BarzaghiF, RoncaroloMG. From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Ann N Y Acad Sci2018; 1417: 5–22.269187969BarzaghiF, Amaya HernandezLC, NevenBet alLong‐term follow‐up of IPEX syndrome patients after different therapeutic strategies: An international multicenter retrospective study. J Allergy Clin Immunol2018; 141: 1036–1049.:e5.2924172910AllanSE, AlstadAN, MerindolNet alGeneration of potent and stable human CD4+ T regulatory cells by activation‐independent expression of FOXP3. Mol Ther2008; 16: 194–202.1798497611PasseriniL, Rossi MelE, SartiranaCet alCD4+ T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci Transl Med2013; 5: 215ra174.12GoodwinM, LeeE, LakshmananUet alCRISPR‐based gene editing enables FOXP3 gene repair in IPEX patient cells. Sci Adv2020; 6: eaaz0571.3249470713BhairavabhotlaR, KimYC, GlassDDet alTranscriptome profiling of human FoxP3+ regulatory T cells. Hum Immunol2016; 77: 201–213.2668641214ZhangY, MaksimovicJ, NaselliGet alGenome‐wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood2013; 122: 2823–2836.2397420315GerrietsVA, KishtonRJ, JohnsonMOet alFoxp3 and Toll‐like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol2016; 17: 1459–1466.2769500316ArveyA, van der VeekenJ, SamsteinRM, FengY, StamatoyannopoulosJA, RudenskyAY. Inflammation‐induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol2014; 15: 580–587.2472835117MacDonaldKN, PiretJM, LevingsMK. Methods to manufacture regulatory T cells for cell therapy. Clin Exp Immunol2019; 197: 52–63.3091330218BondanzaA, ValtolinaV, MagnaniZet alSuicide gene therapy of graft‐versus‐host disease induced by central memory human T lymphocytes. Blood2006; 107: 1828–1836.1629360119HannonM, LechanteurC, LucasSet alInfusion of clinical‐grade enriched regulatory T cells delays experimental xenogeneic graft‐versus‐host disease. Transfusion2014; 54: 353–363.2377268520MutisT, van RijnRS, SimonettiERet alHuman regulatory T cells control xenogeneic graft‐versus‐host disease induced by autologous T cells in RAG2‐/‐γc‐/‐ immunodeficient mice. Clin Cancer Res2006; 12: 5520–5525.1700068821HaruiA, KiertscherSM, RothMD. Reconstitution of huPBL‐NSG mice with donor‐matched dendritic cells enables antigen‐specific T‐cell activation. J Neuroimmune Pharmacol2011; 6: 148–157.2053264722LocafaroG, AndolfiG, RussoFet alIL‐10‐engineered human CD4+ Tr1 cells eliminate myeloid leukemia in an HLA class I‐dependent mechanism. Mol Ther2017; 25: 2254–2269.2880756923McMurchyAN, GilliesJ, AllanSEet alPoint mutants of forkhead box P3 that cause immune dysregulation, polyendocrinopathy, enteropathy, X‐linked have diverse abilities to reprogram T cells into regulatory T cells. J Allergy Clin Immunol2010; 126: 1242–1251.2103638724BaronU, FloessS, WieczorekGet alDNA demethylation in the human FOXP3 locus. discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur J Immunol2007; 37, 2378–2389.1769457525CharbonnierLM, CuiY, Stephen‐VictorEet alFunctional reprogramming of regulatory T cells in the absence of Foxp3. Nat Immunol2019; 20: 1208–1219.3138405726GoldingA, DarkoS, WylieWH, DouekDC, ShevachEM. Deep sequencing of the TCR‐ β repertoire of human forkhead box protein 3 (FoxP3)+ and FoxP3– T cells suggests that they are completely distinct and non‐overlapping. Clin Exp Immunol2017; 188: 12–21.2788097427AhmadzadehMet alTumor‐infiltrating human CD4+ regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Sci Immunol2019; 4: eaao4310.3063535528BabonJA, DeNicolaME, BlodgettDMet alAnalysis of self‐antigen specificity of islet‐infiltrating T cells from human donors with type 1 diabetes. Nat Med2016; 22: 1482–1487.2779861429JelcicI, Al NimerF, WangJet alMemory B cells activate brain‐homing, autoreactive CD4+ T cells in multiple sclerosis. Cell2018; 175: 85–100.e23.3017391630HahnSA, BellinghausenI, TrinschekB, BeckerC. Translating Treg therapy in humanized mice. Front Immunol2015; 6: 623.2669701731MuulLM, TuschongLM, SoenenSLet alPersistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long‐term results of the first clinical gene therapy trial. Blood2003; 101: 2563–2569.1245649632Di IanniM, FalzettiF, CarottiAet alTregs prevent GVHD and promote immune reconstitution in HLA‐haploidentical transplantation. Blood2011; 117: 3921–3928.2129277133BrunsteinCG, MillerJS, CaoQet alInfusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood2011; 117: 1061–1070.2095268734BrunsteinCG, MillerJS, McKennaDHet alUmbilical cord blood‐derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood2016; 127: 1044–1051.2656313335GoettelJA, BiswasS, LexmondWSet alFatal autoimmunity in mice reconstituted with human hematopoietic stem cells encoding defective FOXP3. Blood2015; 125: 3886–3895.2583396436MoesN, Rieux‐LaucatF, BegueBet alReduced expression of FOXP3 and regulatory T‐cell function in severe forms of early‐onset autoimmune enteropathy. Gastroenterology2010; 139: 770–778.2053799837AbelS, LuckheideN, WestendorfAMet alStrong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell‐derived IL‐10 on pathogen clearance during Plasmodium yoelii infection. J Immunol2012; 188: 5467–5477.2254493138KurupSP, Obeng‐AdjeiN, AnthonySMet alRegulatory T cells impede acute and long‐term immunity to blood‐stage malaria through CTLA‐4. Nat Med2017; 23: 1220–1225.2889206539JoshiNS, Akama‐GarrenEH, LuYet alRegulatory T cells in tumor‐associated tertiary lymphoid structures suppress anti‐tumor T cell responses. Immunity2015; 43: 579–590.2634140040TogashiY, ShitaraK, NishikawaH. Regulatory T cells in cancer immunosuppression ‐ implications for anticancer therapy. Nat Rev Clin Oncol2019; 16: 356–371.30705439
Figures






References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995; 155: 1151–1164. - PubMed
-
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10: 490–500. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330–336. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
