Targeting Senescent Cells for a Healthier Aging: Challenges and Opportunities
- PMID: 33304768
- PMCID: PMC7709980
- DOI: 10.1002/advs.202002611
Targeting Senescent Cells for a Healthier Aging: Challenges and Opportunities
Abstract
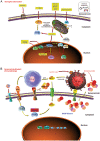
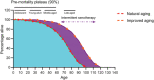
Aging is a physiological decline in both structural homeostasis and functional integrity, progressively affecting organismal health. A major hallmark of aging is the accumulation of senescent cells, which have entered a state of irreversible cell cycle arrest after experiencing inherent or environmental stresses. Although cellular senescence is essential in several physiological events, it plays a detrimental role in a large array of age-related pathologies. Recent biomedical advances in specifically targeting senescent cells to improve healthy aging, or alternatively, postpone natural aging and age-related diseases, a strategy termed senotherapy, have attracted substantial interest in both scientific and medical communities. Challenges for aging research are highlighted and potential avenues that can be leveraged for therapeutic interventions to control aging and age-related disorders in the current era of precision medicine.
Keywords: aging; clinical trials; healthspan; senescent cells; senolytics; senotherapy.
© 2020 The Authors. Published by Wiley‐VCH GmbH.
Conflict of interest statement
J.L.K. has a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
