Precise delivery of obeticholic acid via nanoapproach for triggering natural killer T cell-mediated liver cancer immunotherapy
- PMID: 33304784
- PMCID: PMC7715527
- DOI: 10.1016/j.apsb.2020.09.004
Precise delivery of obeticholic acid via nanoapproach for triggering natural killer T cell-mediated liver cancer immunotherapy
Abstract
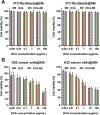
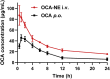
Primary bile acids were reported to augment secretion of chemokine (C‒X‒C motif) ligand 16 (CXCL16) from liver sinusoidal endothelial cells (LSECs) and trigger natural killer T (NKT) cell-based immunotherapy for liver cancer. However, abundant expression of receptors for primary bile acids across the gastrointestinal tract overwhelms the possibility of using agonists against these receptors for liver cancer control. Taking advantage of the intrinsic property of LSECs in capturing circulating nanoparticles in the circulation, we proposed a strategy using nanoemulsion-loaded obeticholic acid (OCA), a clinically approved selective farnesoid X receptor (FXR) agonist, for precisely manipulating LSECs for triggering NKT cell-mediated liver cancer immunotherapy. The OCA-nanoemulsion (OCA-NE) was prepared via ultrasonic emulsification method, with a diameter of 184 nm and good stability. In vivo biodistribution studies confirmed that the injected OCA-NE mainly accumulated in the liver and especially in LSECs and Kupffer cells. As a result, OCA-NE treatment significantly suppressed hepatic tumor growth in a murine orthotopic H22 tumor model, which performed much better than oral medication of free OCA. Immunologic analysis revealed that the OCA-NE resulted in augmented secretion of CXCL16 and IFN-γ, as well as increased NKT cell populations inside the tumor. Overall, our research provides a new evidence for the antitumor effect of receptors for primary bile acids, and should inspire using nanotechnology for precisely manipulating LSECs for liver cancer therapy.
Keywords: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CDCA, chenodeoxycholic acid; Cr, creatinine; FXR, farnesoid X receptor; Farnesoid X receptor; H&E, hematoxylin and eosin; HCC, hepatocellular carcinoma; HPLC, high-performance liquid chromatography; HSCs, hepatic stellate cells; IFN-γ, interferon-γ; IVIS, in vivo imaging system; LSECs, liver sinusoidal endothelial cells; Liver cancer; Liver sinusoidal endothelial cells; NE, nanoemulsion; NKT cells, natural killer T cells; Nanoemulsion; OCA, obeticholic acid; Obeticholic acid; PBC, primary biliary cholangitis; TACE, transarterial chemoembolisation; TSR, tumor suppression rate.
© 2020 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures






References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Chen W.Q., Li H., Sun K.X., Zheng R.S., Zhang S.W., Zeng H.M. Report of cancer incidence and mortality in China, 2014. Chin J Oncol. 2018;40:5–13. - PubMed
-
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Song T.J., Ip E.W., Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248–S260. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

