Clinical outcomes in elderly patients with Morquio a syndrome receiving enzyme replacement therapy - experience in a Colombian center
- PMID: 33304816
- PMCID: PMC7718482
- DOI: 10.1016/j.ymgmr.2020.100679
Clinical outcomes in elderly patients with Morquio a syndrome receiving enzyme replacement therapy - experience in a Colombian center
Abstract
Introduction: Mucopolysaccharidosis type IV A (MPS IVA) or Morquio A syndrome is an autosomal recessive lysosomal storage disease caused by GALNS gene mutations that lead to a deficiency of the N-acetylgalactosamine-6-sulfate sulfatase enzyme and the accumulation of two glycosaminoglycans in cell lysosomes, namely, chondroitin and keratan sulfate.
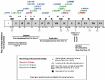
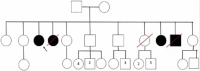
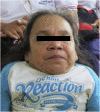
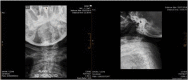
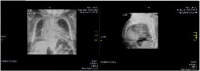
Objective: To present two female patients with Morquio A syndrome in their late adult years (over 50 years of age) with a classical phenotype, treated with enzyme replacement therapy; and to present a summary of the natural history and the characteristics of the disease, and the benefit of comprehensive management.
Materials and methods: Descriptive clinical study before and after the treatment with enzyme replacement therapy as part of the comprehensive management of MPS IVA.
Results: Enzyme replacement therapy with elosulfase alfa was effective, with an adequate safety profile in these two patients, showing evidence of sustained improvement in terms of endurance and gait patterns.
Conclusion: We present two cases of MPS IVA, with longer survival than reported previously in classical phenotypes associated with this disease condition. There is a paucity of reports of similar cases in the literature. We believe that the clinical heterogeneity of the disease manifesting with the classical phenotype, together with comprehensive management, have played a role in the survival of these two patients. Therapy with elosulfase alfa as part of comprehensive management has been crucial; we suspect a clinical response and infer a better quality of life and reduced burden for the caregiver, supporting its use in older patients.
Keywords: Enzyme replacement therapy; Morquio A syndrome; Mucopolysaccharidosis IV A.
© 2020 The Authors.
Conflict of interest statement
None.
Figures



Similar articles
-
Elosulfase Alfa: a review of its use in patients with mucopolysaccharidosis type IVA (Morquio A syndrome).BioDrugs. 2014 Oct;28(5):465-75. doi: 10.1007/s40259-014-0108-z. BioDrugs. 2014. PMID: 25200032 Review.
-
Elosulfase alfa (BMN 110) for the treatment of mucopolysaccharidosis IVA (Morquio A Syndrome).Expert Rev Clin Pharmacol. 2016 Dec;9(12):1521-1532. doi: 10.1080/17512433.2017.1260000. Epub 2016 Nov 23. Expert Rev Clin Pharmacol. 2016. PMID: 27855521 Review.
-
Elosulfase alfa.Drugs Today (Barc). 2014 Jul;50(7):475-83. doi: 10.1358/dot.2014.50.7.2177904. Drugs Today (Barc). 2014. PMID: 25101330 Review.
-
Immunogenicity of Elosulfase Alfa, an Enzyme Replacement Therapy in Patients With Morquio A Syndrome: Results From MOR-004, a Phase III Trial.Clin Ther. 2015 May 1;37(5):1012-1021.e6. doi: 10.1016/j.clinthera.2014.11.005. Epub 2014 Dec 6. Clin Ther. 2015. PMID: 25487082 Clinical Trial.
-
Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA.Mol Genet Metab. 2013 Sep-Oct;110(1-2):54-64. doi: 10.1016/j.ymgme.2013.04.002. Epub 2013 Apr 10. Mol Genet Metab. 2013. PMID: 23665161 Free PMC article. Review.
Cited by
-
Molecular basis of mucopolysaccharidosis IVA (Morquio A syndrome): A review and classification of GALNS gene variants and reporting of 68 novel variants.Hum Mutat. 2021 Nov;42(11):1384-1398. doi: 10.1002/humu.24270. Epub 2021 Aug 23. Hum Mutat. 2021. PMID: 34387910 Free PMC article. Review.
-
Lysosomal storage disorders identified in adult population from India: Experience of a tertiary genetic centre and review of literature.JIMD Rep. 2024 Jan 2;65(2):85-101. doi: 10.1002/jmd2.12407. eCollection 2024 Mar. JIMD Rep. 2024. PMID: 38444573 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

