Cytokinesis-block micronucleus assay of celecoxib and celecoxib derivatives
- PMID: 33304828
- PMCID: PMC7708851
- DOI: 10.1016/j.toxrep.2020.11.004
Cytokinesis-block micronucleus assay of celecoxib and celecoxib derivatives
Abstract
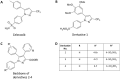
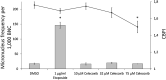
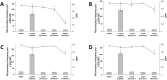
Celecoxib is used widely for the acute treatment of pain and for pain relief in various diseases. Furthermore, it shows potential in chemoprevention, although chronic treatment with celecoxib could lead to adverse effects like cardiovascular events. New derivatives of celecoxib were synthesised that may be suitable as chemopreventive agent without inducing adverse effects. Critical endpoint for a safe use of pharmaceuticals is genotoxicity after application. A standard test for the assessment of genotoxicity is the cytokinesis-block micronucleus assay, that evaluates the number micronuclei after treatment of cells with a test compound as biomarker for DNA damage. Various promising derivatives of celecoxib have been assessed with the cytokinesis-block micronucleus assay in HeLa-H2B-GFP cells. It could be demonstrated, that neither celecoxib nor its derivatives were genotoxic in this assay and therefore celecoxib derivatives could be developed further for a safe use as chemopreventive agent.
Keywords: Celecoxib; Chemoprevention; DNA damage; Micronucleus test.
© 2020 The Author(s).
Conflict of interest statement
The authors report no declarations of interest.
Figures
Similar articles
-
Successful proof of concept of a micronucleus genotoxicity assay on reconstructed epidermis exhibiting intrinsic metabolic activity.Mutat Res Genet Toxicol Environ Mutagen. 2018 May-Jun;829-830:75-86. doi: 10.1016/j.mrgentox.2018.03.004. Epub 2018 Mar 10. Mutat Res Genet Toxicol Environ Mutagen. 2018. PMID: 29704997
-
Contribution of ionic silver to genotoxic potential of nanosilver in human liver HepG2 and colon Caco2 cells evaluated by the cytokinesis-block micronucleus assay.J Appl Toxicol. 2016 Apr;36(4):532-42. doi: 10.1002/jat.3279. Epub 2016 Jan 27. J Appl Toxicol. 2016. PMID: 26813850
-
Validity of the Lymphocyte Cytokinesis-Block Micronucleus Assay (L-CBMN) as biomarker for human exposure to chemicals with different modes of action: A synthesis of systematic reviews.Mutat Res Genet Toxicol Environ Mutagen. 2018 Dec;836(Pt A):47-52. doi: 10.1016/j.mrgentox.2018.05.010. Epub 2018 May 7. Mutat Res Genet Toxicol Environ Mutagen. 2018. PMID: 30389162
-
Does the recommended lymphocyte cytokinesis-block micronucleus assay for human biomonitoring actually detect DNA damage induced by occupational and environmental exposure to genotoxic chemicals?Mutagenesis. 2013 Jul;28(4):375-80. doi: 10.1093/mutage/get026. Epub 2013 May 3. Mutagenesis. 2013. PMID: 23644166 Review.
-
Inclusion of micronuclei in non-divided mononuclear lymphocytes and necrosis/apoptosis may provide a more comprehensive cytokinesis block micronucleus assay for biomonitoring purposes.Mutagenesis. 2001 Jan;16(1):51-8. doi: 10.1093/mutage/16.1.51. Mutagenesis. 2001. PMID: 11139598 Review.
Cited by
-
Fate of micronuclei and micronucleated cells after treatment of HeLa cells with different genotoxic agents.Arch Toxicol. 2023 Mar;97(3):875-889. doi: 10.1007/s00204-022-03433-9. Epub 2022 Dec 23. Arch Toxicol. 2023. PMID: 36564592 Free PMC article.
References
-
- Cohen B., Preuss C.V. StatPearls Publishing StatPearls Publishing LLC; Treasure Island (FL): 2020. Celecoxib. StatPearls.
-
- Saxena P., Sharma P.K., Purohit P. A journey of celecoxib from pain to cancer. Prostaglandins Other Lipid Mediat. 2019;147 - PubMed
LinkOut - more resources
Full Text Sources

