Cytokinesis-block micronucleus assay of celecoxib and celecoxib derivatives
- PMID: 33304828
- PMCID: PMC7708851
- DOI: 10.1016/j.toxrep.2020.11.004
Cytokinesis-block micronucleus assay of celecoxib and celecoxib derivatives
Abstract
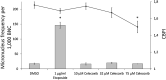
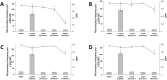
Celecoxib is used widely for the acute treatment of pain and for pain relief in various diseases. Furthermore, it shows potential in chemoprevention, although chronic treatment with celecoxib could lead to adverse effects like cardiovascular events. New derivatives of celecoxib were synthesised that may be suitable as chemopreventive agent without inducing adverse effects. Critical endpoint for a safe use of pharmaceuticals is genotoxicity after application. A standard test for the assessment of genotoxicity is the cytokinesis-block micronucleus assay, that evaluates the number micronuclei after treatment of cells with a test compound as biomarker for DNA damage. Various promising derivatives of celecoxib have been assessed with the cytokinesis-block micronucleus assay in HeLa-H2B-GFP cells. It could be demonstrated, that neither celecoxib nor its derivatives were genotoxic in this assay and therefore celecoxib derivatives could be developed further for a safe use as chemopreventive agent.
Keywords: Celecoxib; Chemoprevention; DNA damage; Micronucleus test.
© 2020 The Author(s).
Conflict of interest statement
The authors report no declarations of interest.
Figures
References
-
- Cohen B., Preuss C.V. StatPearls Publishing StatPearls Publishing LLC; Treasure Island (FL): 2020. Celecoxib. StatPearls.
-
- Saxena P., Sharma P.K., Purohit P. A journey of celecoxib from pain to cancer. Prostaglandins Other Lipid Mediat. 2019;147 - PubMed
LinkOut - more resources
Full Text Sources

