Protective multi-epitope candidate vaccine for urinary tract infection
- PMID: 33304840
- PMCID: PMC7711219
- DOI: 10.1016/j.btre.2020.e00564
Protective multi-epitope candidate vaccine for urinary tract infection
Abstract
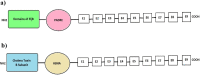
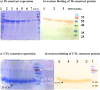
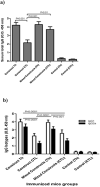
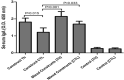
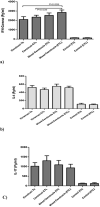
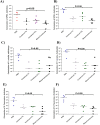
Urinary tract infections (UTIs) are induced by exogenous organisms including extraintestinal pathogenic such as Escherichia coli (ExPEC), Proteus mirabilis and Klebsiella pneumonia, which are closely related. These organisms can colonize in the urinary tract and cause UTIs. In this study, a cross-reactive multi-epitope vaccine was designed by two constructs to stimulate the immune system (CD8+ and CD4 + T cells) against ExPEC, Proteus mirabilis and Klebsiella pneumonia strains. Uropathogenic Escherichia coli (UPEC), Proteus mirabilis and Klebsiella pneumoniae are the main bacterial cause of UTI. They were used for designing experimental candidate vaccine, and their immunogenicity and protectivity were assessed. In this study, conserved antigens from their bacterial genomes were considered, and informatics-based immunological vaccine with cross-protective T and B-cells epitopes was designed and evaluated. The vaccine candidate was used as a broad immune system inducer, and its cross-protective immunity and protectivity were confirmed in in vivo experiments.
Keywords: Escherichia coli (ExPEC); Klebsiella pneumonia; Multi-epitope vaccine; Proteus mirabilis; Urinary tract infections (UTIs).
© 2020 Published by Elsevier B.V.
Figures
References
-
- Foxman B. Women Heal. second ed. Elsevier; 2013. Urinary tract infection; pp. 553–564.
-
- Ayub M., Amir S., Firdous K., Khan S., Iqbal I. E. coli the most prevalent causative agent urinary tract infection in pregnancy: comparative analysis of susceptibility and resistance pattern of antimicrobials. Arch. Clin. Microbiol. 2016;7:4.
-
- Chen C.Y., Chen C.Y.H., Lu P.L., Lin W.R., Chen T.C., Lin C.Y. Proteus mirabilis urinary tract infection and bacteremia: risk factors, clinical presentation, and outcomes. J. Microbiol. Immunol. Infect. 2012;45:228–236. - PubMed
-
- Foris L., Snowden J. 2017. Proteus Mirabilis Infections. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
