CDKN2A and MTAP Are Useful Biomarkers Detectable by Droplet Digital PCR in Malignant Pleural Mesothelioma: A Potential Alternative Method in Diagnosis Compared to Fluorescence In Situ Hybridisation
- PMID: 33304846
- PMCID: PMC7693432
- DOI: 10.3389/fonc.2020.579327
CDKN2A and MTAP Are Useful Biomarkers Detectable by Droplet Digital PCR in Malignant Pleural Mesothelioma: A Potential Alternative Method in Diagnosis Compared to Fluorescence In Situ Hybridisation
Abstract
Background: The diagnosis of malignant pleural mesothelioma (MPM) can be difficult, in part due to the difficulty in distinguishing between MPM and reactive mesothelial hyperplasia (RMH). The tumor suppressor gene, CDKN2A, is frequently silenced by epigenetic mechanisms in many cancers; in the case of MPM it is mostly silenced via genomic deletion. Co-deletion of the CDKN2A and methylthioadenosine phosphorylase (MTAP) genes has been researched extensively and discovered to be a highly specific characteristic of MPM. Most studies have used FISH to detect the deletion of CDKN2A and IHC for MTAP as a surrogate for this. In this study, we aim to investigate and validate droplet digital PCR (ddPCR) as an emerging alternative and efficient testing method in diagnosing MPM, by particularly emphasizing on the loss of MTAP and CDKN2A.
Methods: This study included 75 formalin fixed paraffin embedded (FFPE) MPM tissue, and 12 normal pleural tissue and 10 RMH as control. Additionally, primary MPM cell lines and normal pleural samples were used as biomarker detection controls, as established in our previous publication. All FFPE specimens were processed to isolate the DNA, that was subsequently used for ddPCR detection of CDKN2A and MTAP. FFPE samples were also analyzed by fluorescence in situ hybridization (FISH) for CDKN2A and MTAP deletion, and for MTAP IHC expression. Concordance of IHC and ddPCR with FISH were studied in these samples.
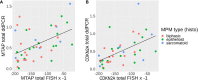
Results: 95% and 82% of cases showed co-deletion of both MTAP and CDKN2A when determined by FISH and ddPCR respectively. ddPCR has a sensitivity of 72% and specificity of 100% in detecting CDKN2A homozygous loss in MPM. ddPCR also has a concordance rate of 92% with FISH in detecting homozygous loss of CDKN2A. MTAP IHC was 68% sensitive and 100% specific for detecting CDKN2A homozygous loss in MPM when these losses were determined by ddPCR.
Conclusion: Our study confirms that MTAP is often co-deleted with CDKN2A in MPM. Our in-house designed ddPCR assays for MTAP and CDKN2A are useful in differentiating MPM from RMH, and is highly concordant with FISH that is currently used in diagnosing MPM. ddPCR detection of these genetic losses can potentially be utilized as an alternative method in the diagnosis of MPM and for the future development of a less-invasive MPM-specific detection technique on MPM tumor tissue DNA.
Keywords: CDKN2A; droplet digital PCR; fluorescence in situ hybridization; malignant pleural mesothelioma; methylthioadenosine phosphorylase.
Copyright © 2020 Cheng, Yuen, Rath, Johnson, Zhuang, Yu, Aleksova, Linton, Kao, Clarke, McCaughan, Takahashi and Lee.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

