Searching for COVID-19 Antibodies in Czech Children-A Needle in the Haystack
- PMID: 33304869
- PMCID: PMC7693718
- DOI: 10.3389/fped.2020.597736
Searching for COVID-19 Antibodies in Czech Children-A Needle in the Haystack
Abstract
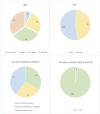
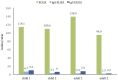
During the COVID-19 pandemics of 2020, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), both adults and children were shown to mount a specific antibody response to the virus. As infected children often exhibit mild symptoms or even remain asymptomatic, they are likely to be under tested for the direct presence of the virus. Mapping the SARS-CoV-2 antibodies frequency informs more accurately on the disease prevalence and helps guide the protective and therapeutic strategies. To date, only few seroprevalence studies included children. In the Czech Republic, in April 2020, the overall SARS-CoV-2 seroprevalence was estimated not to exceed 1.3%. In July and August, 2020, we screened 200 children (0-18 years of age), who attended the pediatric department of a large hospital in Prague for various COVID-19-unrelated reasons, for the presence of SARS-CoV-2 antibodies. Zero seropositive subjects were found. Therefore, we hereby report a low (<0.5%) seroprevalence amongst children in Prague, as of August, 2020.
Keywords: COVID-19; SARS-CoV-2; antibodies; children; pediatric; seroprevalence.
Copyright © 2020 Bloomfield, Pospisilova, Cabelova, Sediva, Ibrahimova, Borecka and Magner.
Figures
References
-
- COVID-19 Prehled aktuální situace v CR (COVID-19 Overview of Current Situation in the Czech Republic. 2020). (2020). Available online at: https://onemocneni-aktualne.mzcr.cz/covid-19 (accessed August 8, 2020).
-
- European Center for Disease Control (2020). Available online at: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed August 8, 2020).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

