Chiral-at-Metal: Iridium(III) Tetrazole Complexes With Proton-Responsive P-OH Groups for CO2 Hydrogenation
- PMID: 33304883
- PMCID: PMC7692406
- DOI: 10.3389/fchem.2020.591353
Chiral-at-Metal: Iridium(III) Tetrazole Complexes With Proton-Responsive P-OH Groups for CO2 Hydrogenation
Abstract
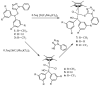
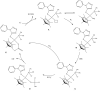
A rise in atmospheric CO2 levels, following years of burning fossil fuels, has brought about increase in global temperatures and climate change due to the greenhouse effect. As such, recent efforts in addressing this problem have been directed to the use of CO2 as a non-expensive and non-toxic single carbon, C1, source for making chemical products. Herein, we report on the use of tetrazolyl complexes as catalyst precursors for hydrogenation of CO2. Specifically, tetrazolyl compounds bearing P-S bonds have been synthesized with the view of using these as P∧N bidentate tetrazolyl ligands (1-3) that can coordinate to iridium(III), thereby forming heteroatomic five-member complexes. Interestingly, reacting the P,N'-bidentate tetrazolyl ligands with [Ir(C5 Me 5)Cl 2]2 led to serendipitous isolation of chiral-at-metal iridium(III) half-sandwich complexes (7-9) instead. Complexes 7-9 were obtained via prior formation of non-chiral iridium(III) half-sandwich complexes (4-6). The complexes undergo prior P-S bond heterolysis of the precursor ligands, which then ultimately results in new half-sandwich iridium(III) complexes featuring monodentate phosphine co-ligands with proton-responsive P-OH groups. Conditions necessary to significantly affect the rate of P-S bond heterolysis in the precursor ligand and the subsequent coordination to iridium have been reported. The complexes served as catalyst precursors and exhibited activity in CO2 and bicarbonate hydrogenation in excellent catalytic activity, at low catalyst loadings (1 μmol or 0.07 mol% with respect to base), producing concentrated formate solutions (ca 180 mM) exclusively. Catalyst precursors with proton-responsive P-OH groups were found to influence catalytic activity when present as racemates, while ease of dissociation of the ligand from the iridium center was observed to influence activity in spite of the presence of electron-donating ligands. A test for homogeneity indicated that hydrogenation of CO2 proceeded by homogeneous means. Subsequently, the mechanism of the reaction by the iridium(III) catalyst precursors was studied using 1H NMR techniques. This revealed that a chiral-at-metal iridium hydride species generated in situ served as the active catalyst.
Keywords: CO2 Hydrogenation 4; CO2 utilization; NaHCO3 reduction; chiral-at-metal 1; green chemistry; iridium(III) complexes 3; tetrazole 2.
Copyright © 2020 Ocansey, Darkwa and Makhubela.
Figures



References
-
- Ahmad G., Rasool N., Rizwan K., Altaf A. A., Rashid U., Hussein M. Z., et al. (2019). Role of pyridine nitrogen in palladium-catalyzed imine hydrolysis: a case study of (E)-1-(3-bromothiophen- 2-yl)-N-(4-methylpyridin-2-yl)methanimine. Molecules 24, 2609. 10.3390/molecules24142609 - DOI - PMC - PubMed
-
- Boddien A., Federsel C., Sponholz P., Mellmann D., Jackstell R., Junge H., et al. (2012). Towards the development of a hydrogen battery. Energy Environ. Sci. 5, 8907–8911. 10.1039/c2ee22043a - DOI
LinkOut - more resources
Full Text Sources
Research Materials

