Evaluation of Hydrogen Peroxide Fumigation and Heat Treatment for Standard Emergency Arthropod Inactivation in BSL-3 Insectaries
- PMID: 33304894
- PMCID: PMC7701145
- DOI: 10.3389/fbioe.2020.602937
Evaluation of Hydrogen Peroxide Fumigation and Heat Treatment for Standard Emergency Arthropod Inactivation in BSL-3 Insectaries
Abstract
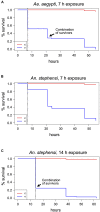
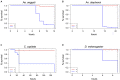
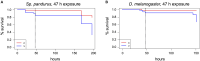
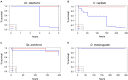
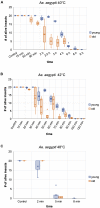
Climate change and global movements of people and goods have accelerated the spread of invasive species, including insects that vector infectious diseases, which threaten the health of more than half of the world's population. Increasing research efforts to control these diseases include the study of vector - pathogen interactions, involving the handling of pathogen-infected vector insects under biosafety level (BSL) 2 and 3 conditions. Like microbiology BSL-3 laboratories, BSL-3 insectaries are usually subjected to fixed-term or emergency room decontamination using recognized methods such as hydrogen peroxide (H2O2) or formaldehyde fumigation. While these procedures have been standardized and approved for the inactivation of diverse pathogens on surfaces, to date, there are no current standards for effective room-wide inactivation of insects in BSL-3 facilities in case of an emergency such as the accidental release of a large number of infected vectors. As H2O2 is often used for standard room decontamination in BSL-3 facilities, we evaluated H2O2 fumigation as a potential standard method for the safe, room-wide deactivation of insects in BSL-3 insectaries in comparison to heat treatment. To account for physiological diversity in vector insect species, six species from three different orders were tested. For the H2O2 fumigation we observed a strong but also varying resilience across all species. Lethal exposure time for the tested dipterans ranged from nine to more than 24 h. Furthermore, the coleopteran, Tribolium castaneum, did not respond to continuous H2O2 exposure for 48 h under standard room decontamination conditions. In contrast, temperatures of 50°C effectively killed all the tested species within 2 to 10 min. The response to lower temperatures (40-48°C) again showed a strong variation between species. In summary, results suggest that H2O2 fumigation, especially in cases where a gas generator is part of the laboratory equipment, may be used for the inactivation of selected species but is not suitable as a general emergency insect inactivation method under normal room decontamination conditions. In contrast, heat treatment at 48 to 50°C has the potential to be developed as an approved standard procedure for the effective inactivation of insects in BSL-3 facilities.
Keywords: BSL-3 insectary; containment; gene drive; heat susceptibility; hydrogen peroxide; inactivation procedures; infectious disease; vector insects.
Copyright © 2020 Häcker, Koller, Eichner, Martin, Liapi, Rühl, Rehling and Schetelig.
Figures


Similar articles
-
Opinion: Airtightness for Decontamination by Fumigation of High-Containment Laboratories.Appl Biosaf. 2019 Dec 1;24(4):207-212. doi: 10.1177/1535676019871370. Epub 2019 Dec 1. Appl Biosaf. 2019. PMID: 36032062 Free PMC article.
-
Analysis of Range and Use of a Hybrid Hydrogen Peroxide System for Biosafety Level 3 and Animal Biosafety Level 3 Agriculture Laboratory Decontamination.Appl Biosaf. 2022 Mar 1;27(1):7-14. doi: 10.1089/apb.2021.0012. Epub 2022 Mar 15. Appl Biosaf. 2022. PMID: 36032318 Free PMC article.
-
Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis.Lett Appl Microbiol. 2005;40(6):448-52. doi: 10.1111/j.1472-765X.2005.01683.x. Lett Appl Microbiol. 2005. PMID: 15892741
-
Containment of arthropod disease vectors.ILAR J. 2005;46(1):53-61. doi: 10.1093/ilar.46.1.53. ILAR J. 2005. PMID: 15644564 Review.
-
The Hitchhiker's Guide to Hydrogen Peroxide Fumigation, Part 1: Introduction to Hydrogen Peroxide Fumigation.Appl Biosaf. 2020 Dec 1;25(4):214-224. doi: 10.1177/1535676020921007. Epub 2020 Dec 1. Appl Biosaf. 2020. PMID: 36032396 Free PMC article. Review.
References
-
- Calhoun P. (2019). Unconditional Exact Test. 2.0. Available online at: https://CRAN.R-project.org/package=Exact (accessed March 24, 2020).
-
- Cheney J. E., Collins C. H. (1995). Formaldehyde disinfection in laboratories: limitations and hazards. Br. J. Biomed. Sci. 52 195–201. - PubMed
-
- Council of the European Union (1990). Council Directive 90/679/EEC of 26 November 1990 on the protection of workers from risks related to exposure to biological agents at work (seventh individual Directive within the meaning of Article 16 (1) of Directive 89/391/EEC). Luxemburg: Publications office of the European Union.
LinkOut - more resources
Full Text Sources
Research Materials

